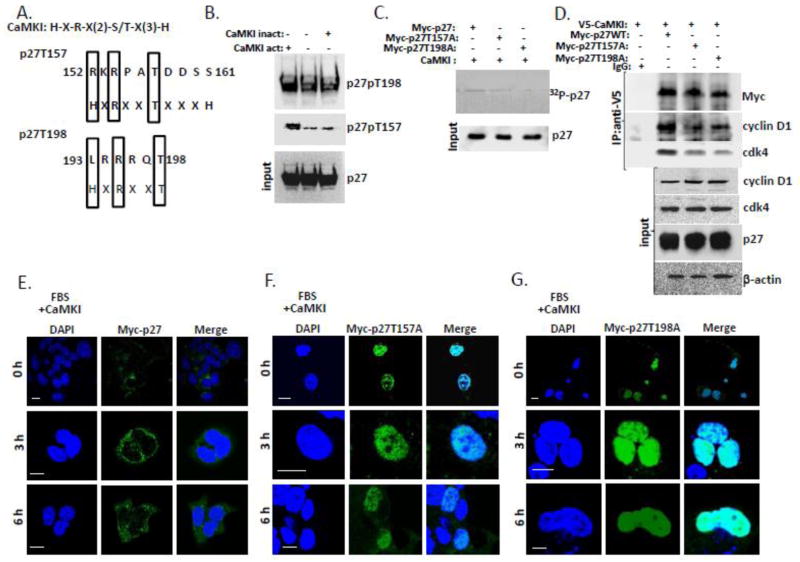
Figure 6. CaMKI phosphorylates human p27 and regulates its intracellular localization in human lung epithelia.
(A) Alignment of the CaMKI consensus recognition motif and putative phosphorylation sites within human p27. (B) Purified CaMKI phosphorylates recombinant human p27 at T157 and T198 in vitro. p27 (3μg) was incubated with 10 μCi [γ-32P]ATP in the presence or absence of the active form of CaMKI (500 nM) and heat-inactivated CaMKI form. After 1 h of incubation at 30°, reactions were terminated with 4X Laemmli protein loading buffer and products were resolved by 10% SDS-PAGE and analyzed by immunoblotting using antibodies against total and phospho-specific forms of p27 (p27, T157 and T198). (C) CaMKI phosphorylates p27 WT but not p27 mutants in human lung epithelia. A549 cells were transfected with Myc-tagged p27 WT or p27 point mutants p27 T157A or p27 T198A. Myc-tagged proteins were then pulled down with Myc-agarose. The Myc-p27-, Myc-p27T157A- and Myc-p27T198A- beads were first dephosphorylated using alkaline phosphatase (5 U) followed by incubation with the active form of CaMKI (500nM) in the presence of 10 μCi [γ-32P]ATP and Ca2+ (2 mM). After 1 h of incubation at 30°, reaction products were resolved by 10% SDS-PAGE and analyzed by autoradiography and immunoblotting. (D) CaMKI induces binding of cdk4 and cyclin D1 with wild type of p27 but not with the p27 point mutants. A549 cells were co-transfected with V5-CaMKI and either Myc-p27, Myc-p27 T157A or Myc-p27 T198A plasmids followed by immunoprecipitation with V5 antibody. Immunoprecipitants and input were analyzed by immunoblotting using an appropriate antibody. (E–G) A549 cells were co-transfected with CaMKI and either Myc-p27 (E), Myc-p27T157A (F) or Myc-p27T198A plasmids (G). Cells were then synchronized by serum starvation and released in 48 h by addition of 10% FBS followed by fixation at indicated times and immunostaining using anti-Myc antibody and DAPI to visualize nuclei. The data from each panel represents at least n=3 separate experiments. Scale bar, 10 μm.