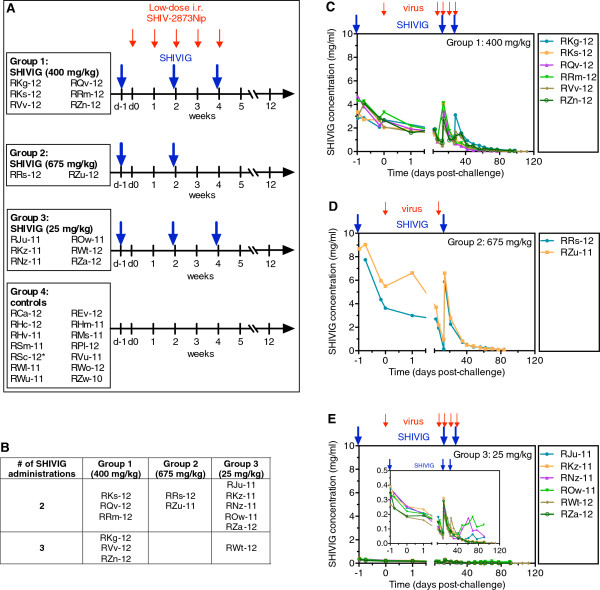
Figure 3.
Study design and SHIVIG pharmacokinetics. A. Animal study design and timeline. Four groups of female RMs were enrolled. Group 1 (n = 6) received 400 mg/kg of SHIVIG, Group 2 (n = 2), 675 mg/kg, and Group 3 (n = 6), 25 mg/kg, respectively. Group 4 (n = 14) RMs served as virus-only controls. Ten animals were enrolled and four additional animals used for virus titration given the identical virus dose served as additional controls. The challenge virus, SHIV-2873Nip, had been titrated i.r. to yield systemic infection (>104 viral RNA copies) in untreated monkeys after a maximum of 5 weekly challenges (small red arrows); the challenge dose was 5,000 50% tissue culture infectious doses (TCID50) measured by TZM-bl assay. SHIVIG infusions (large blue arrows) as well as viral challenges were stopped after the monkeys became viremic (>104 viral RNA copies/ml). B. The number of SHIVIG administrations that RMs from different groups received while their vRNA loads were <104 copies/ml. C-E. SHIVIG pharmacokinetics in RM groups. Large blue arrows indicate biweekly SHIVIG administrations and small red arrows indicate SHIV-2873Nip challenges; C. Group 1 (400 mg/kg of SHIVIG, n = 6). D. Group 2 (675 mg/kg of SHIVIG, n = 2); E. Group 3 (25 mg/kg of SHIVIG, n = 6). The insert represents the same graph showing the lower Y axis range. Serial dilutions of RM plasmas were incubated on ELISA plates to which HIV-1 BaL gp120 had been added and preimmobilized. Binding was assessed as described in Methods. All samples were assayed in triplicate.