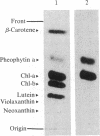
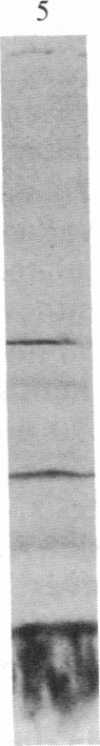
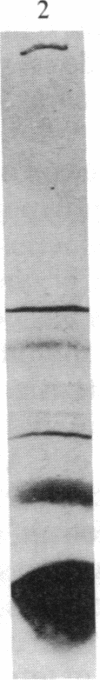
Abstract
Isolated developing plastids from greening cucumber cotyledons or from photoperiodically grown pea seedlings incorporated 14C-labeled 5-aminolevulinic acid (ALA) into chlorophyll (Chl). Incorporation was light dependent, enhanced by S-adenosylmethionine, and linear for 1 hr. The in vitro rate of Chl synthesis from ALA was comparable to the in vivo rate of Chl accumulation. Levulinic acid and dioxoheptanoic acid strongly inhibited Chl synthesis but not plastid protein synthesis. Neither chloramphenicol nor spectinomycin affected Chl synthesis, although protein synthesis was strongly inhibited. Components of thylakoid membranes from plastids incubated with [14C]ALA were resolved by electrophoresis and then subjected to autoradiography. This work showed that (i) newly synthesized Chl was assembled into Chl-protein complexes and (ii) the inhibition of protein synthesis during the incubation did not alter the labeling pattern. Thus, there was no observable short-term coregulation between Chl synthesis (from ALA) and the synthesis of membrane proteins in isolated plastids.
Keywords: 5-aminolevulinic acid, chloroplast development, protein synthesis, photosynthesis
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argyroudi-Akoyunoglou J. H., Akoyunoglou A., Kalosakas K., Akoyunoglou G. Reorganization of the Photosystem II Unit in Developing Thylakoids of Higher Plants after Transfer to Darkness : Changes in Chlorophyll b, Light-Harvesting Chlorophyll Protein Content, and Grana Stacking. Plant Physiol. 1982 Nov;70(5):1242–1248. doi: 10.1104/pp.70.5.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Polypeptide turnover in darkness. Eur J Biochem. 1981 Aug;118(1):61–70. doi: 10.1111/j.1432-1033.1981.tb05486.x. [DOI] [PubMed] [Google Scholar]
- Camm E. L., Green B. R. Widespread distribution of some minor chlorophyll-protein complexes in some plants and algae. Plant Physiol. 1981 May;67(5):1061–1063. doi: 10.1104/pp.67.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Cashmore A., Chua N. H. Nucleotide sequences of two pea cDNA clones encoding the small subunit of ribulose 1,5-bisphosphate carboxylase and the major chlorophyll a/b-binding thylakoid polypeptide. J Biol Chem. 1983 Feb 10;258(3):1399–1402. [PubMed] [Google Scholar]
- Drews G., Peters J., Dierstein R. Molecular-organization and biosynthesis of pigment-protein complexes of Rhodopseudomonas capsulata. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):151–158. doi: 10.1016/s0769-2609(83)80102-1. [DOI] [PubMed] [Google Scholar]
- Fish L. E., Jagendorf A. T. High rates of protein synthesis by isolated chloroplasts. Plant Physiol. 1982 Oct;70(4):1107–1114. doi: 10.1104/pp.70.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fufsler T. P., Castelfranco P. A., Wong Y. S. Formation of Mg-Containing Chlorophyll Precursors from Protoporphyrin IX, delta-Aminolevulinic Acid, and Glutamate in Isolated, Photosynthetically Competent, Developing Chloroplasts. Plant Physiol. 1984 Apr;74(4):928–933. doi: 10.1104/pp.74.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs S. P. The chloroplasts of some algal groups may have evolved from endosymbiotic eukaryotic algae. Ann N Y Acad Sci. 1981;361:193–208. doi: 10.1111/j.1749-6632.1981.tb46519.x. [DOI] [PubMed] [Google Scholar]
- Griffith I. P. Immediate visualization of proteins in dodecyl sulfate-polyacrylamide gels by prestaining with Remazol dyes. Anal Biochem. 1972 Apr;46(2):402–412. doi: 10.1016/0003-2697(72)90313-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell J. P., Thornber J. P., Boggs R. T. Higher plant chloroplasts: Evidence that all the chlorophyll exists as chlorophyll-protein complexes. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1233–1235. doi: 10.1073/pnas.76.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J., Herrmann R. G. Nucleotide sequence of the gene for the P680 chlorophyll alpha apoprotein of the photosystem II reaction center from spinach. Nucleic Acids Res. 1984 Mar 26;12(6):2837–2850. doi: 10.1093/nar/12.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley J. M. Chloroplast evolution--ancient and modern. Ann N Y Acad Sci. 1981;361:154–165. doi: 10.1111/j.1749-6632.1981.tb46517.x. [DOI] [PubMed] [Google Scholar]
- Woo K. C., Gerbaud A., Furbank R. T. Evidence for Endogenous Cyclic Photophosphorylation in Intact Chloroplasts: CO(2) Fixation with Dihydroxyacetone Phosphate. Plant Physiol. 1983 Jun;72(2):321–325. doi: 10.1104/pp.72.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski R. E., Price C. A. Synthesis of thylakoid membrane proteins by chloroplasts isolated from spinach. Cytochrome b559 and P700-chlorophyll a-protein. J Cell Biol. 1980 May;85(2):435–445. doi: 10.1083/jcb.85.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]