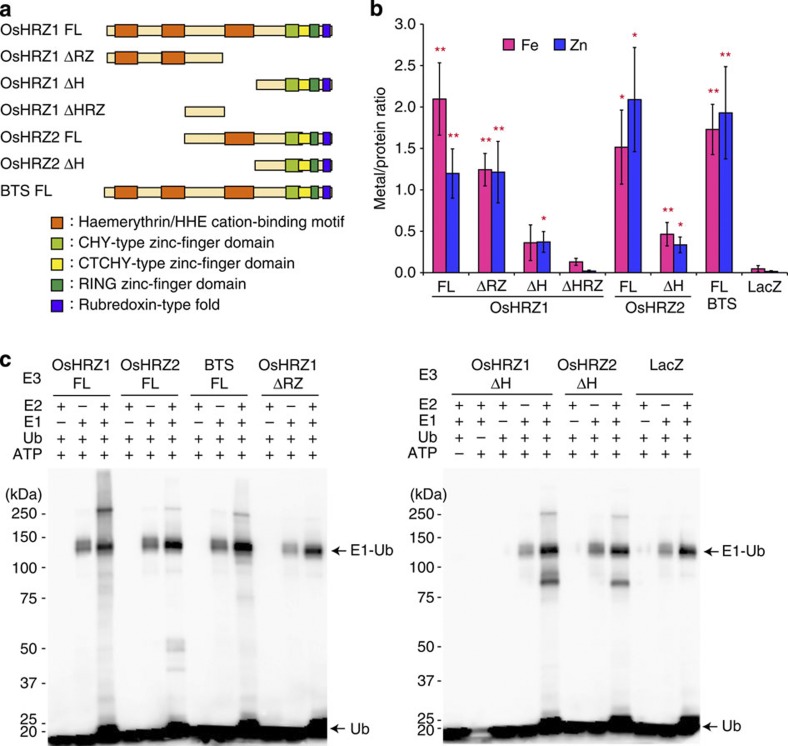
Figure 3. Metal-binding analysis and ubiquitination assay of HRZ and BTS.
(a) Domain structures of the MBP fusions for the HRZ derivatives analysed. (b) Metal concentration analysis. MBP fusions of HRZ derivatives, or MBP-LacZ as a negative control, were expressed in E. coli, purified and subjected to metal concentration measurements by inductively coupled plasma optical emission spectrometry. Shown is the molar ratio of each metal per protein (means±s.e.m. of three to nine replicates derived from one to four biological replicates). Asterisks indicate significant differences from MBP-LacZ (two-sample Student’s t-test; *P<0.05; **P<0.01; Supplementary Table S1). (c) Ubiquitination assay. MBP fusions of HRZ derivatives, or MBP-LacZ as a negative control, were subjected to an in vitro ubiquitination assay with (+) or without (–) ATP, biotinylated ubiquitin (Ub) and human E1 and E2 (UbcH5a). Ubiquitinated proteins were detected by western blotting using a streptavidin–horseradish peroxidase conjugate detection system. The positions and sizes of the molecular mass markers are shown to the left of each blot. E1-ubiquitin (E1-Ub) conjugates and free ubiquitin (Ub) are indicated with arrows.