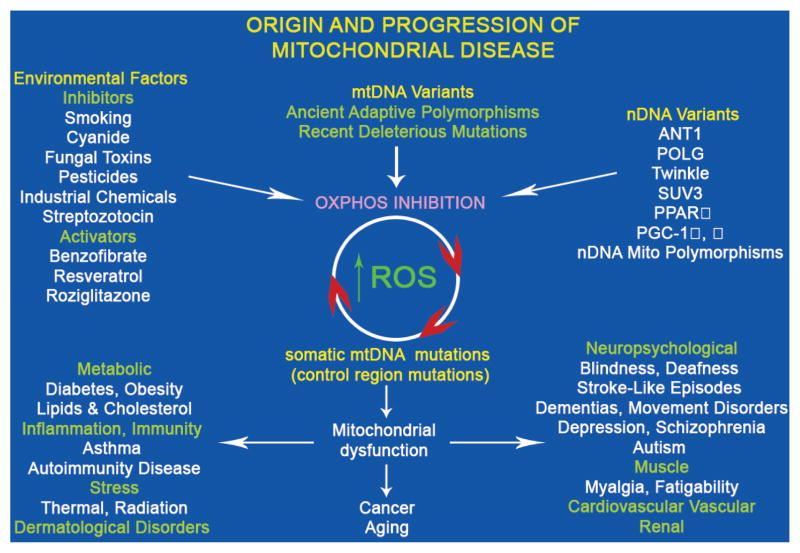
Figure 3.
Classes of human mitochondrial gene mutations in the origin of metabolic and degenerative diseases, cancer, and aging. The “mitochondrial genome” encompasses ~1500 nDNA genes dispersed across the chromosomes plus 37 critical energetic genes within mtDNA. Genetic variation in any of these mitochondrial genes may perturb the mitochondrial OXPHOS. An array of common environmental agents and pharmacological agents can also modulate mitochondrial bioenergetics and/or biogenesis. Inhibition of OXPHOS can increase mitochondrial ROS production, which will damage mtDNA, gradually erode the cellular capacity to generate energy, and create the clock central to aging and adult cancers. OXPHOS dysfunction will have the greatest effect on tissues having the highest energy demand (brain, heart, skeletal muscle, kidney, endocrine system) to cause degenerative diseases. Altered mitochondrial energy production will also perturb caloric sensing and use, resulting in common metabolic diseases such as diabetes and obesity. Finally, altered mitochondrial ROS production and redox biology will precipitate inflammatory disease and change mitochondrial coupling efficiency to affect thermal modulation and sensitivity to radiation-induced cellular toxicity.