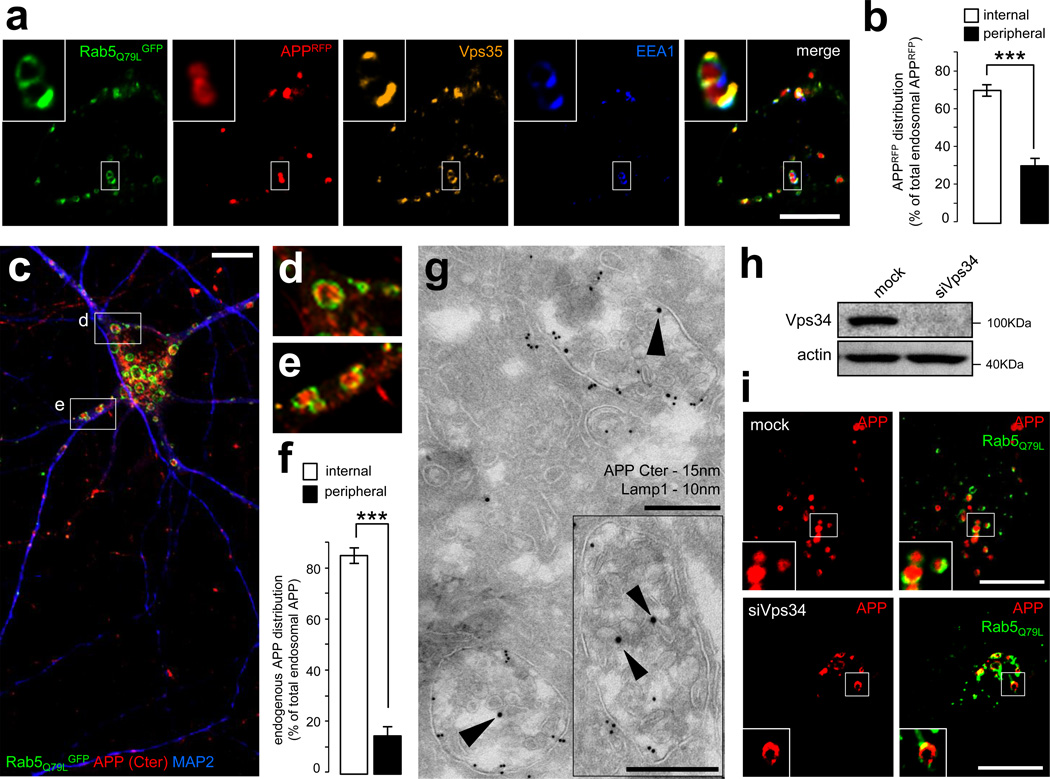
Figure 4. APP is sorted into the intraluminal vesicles of multivesicular endosomes.
a) Confocal analysis of HeLa cells transfected with APPRFP (red) and the dominant-positive Rab5Q79LGFP mutant (green), which was used for its ability to generate giant endosomes. The cells were fixed and labeled with anti-EEA1 (blue) and anti-Vps35 (orange) antibodies. Scale bar = 10µm.
b) Bar diagram showing a quantification of the localization of APPRFP inside the endosomal lumen (internal) vs. on the endosomal limiting membrane (peripheral), expressed as % of the total endosomal APPRFP. Values denote means ± SEM (n = 20 cells from 3 experiments with an average quantification of 15 endosomes per cell). ***, denotes P values < 0.001 from a Student's t-test.
c) Confocal analysis of mouse hippocampal neurons transfected with Rab5Q79LGFP (green) at DIV9 and stained for endogenous APP (using the Cter antibody, red) and MAP2, a marker of the somatodendritic compartment (blue). Two insets are selected and presented in panels (d) and (e). Scale bar = 10 µm.
d) First inset showing a magnification of the area containing enlarged endosomes presented in panel (c).
e) Second inset showing a magnification of the area containing enlarged endosomes presented in panel (c).
f) Bar diagram showing the quantification of the endosomal localization of endogenous APP in neurons, as in panel (b).
g) Immunogold EM analysis of mouse hippocampal neurons at DIV15 in ultrathin cryosections double-labeled with antibodies to endogenous APP COOH-terminal domain (APP C-ter; PAG 15nm, arrowheads), located primarily on the intraluminal vesicles, and LAMP-1 (PAG 10nm). Scale bars = 250 nm.
h) Western blot analysis of endogenous Vps34 and the loading marker actin in HeLa cells transfected with mock siRNA (mock) or siRNA against human Vps34 (siVps34) followed by transfection with APPRFP and Rab5Q79LGFP.
i) Confocal analysis of HeLa cells transfected with mock siRNA (mock, top panel) or siVps34 (bottom panel) for 72h and then split, prior to transfection with APPRFP and Rab5Q79LGFP. Scale bar = 10µm.