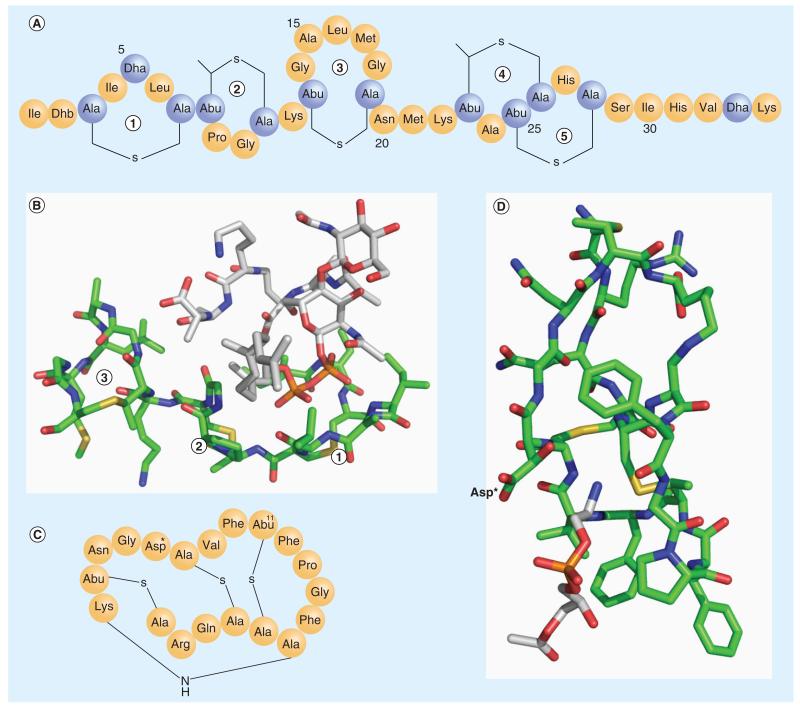
Figure 2. Lipid-targeting lantibiotics.
(A) The primary structure of nisin. (B) The complex structure between nisin and lipid II (PDB: 1WCO). Only the N-terminal fragment (1, 2, 3 rings) of nisin interacts with lipid II. The color scheme used: C of nisin in green, C of lipid II in white, N in blue, O in red, S in yellow, and P in brown. (C) The primary structure of cinnamycin. Asp* represents β-hydroxyaspartate. (D) The NMR structure of the cinnamycin and lyso-phosphatidylethanolamine complex (PDB: 2DDE). The color scheme used: C of cinnamycin in green, C of lyso-phosphatidylethanolamine in white, N in blue, O in red and S in yellow. The lyso-phosphatidylethanolamine headgroup inserts into the hydrophobic pocket of cinnamycin. The key polar residue (Asp*), which interacts with the phosphatidylethanolamine headgroup, is labeled.
Abu: α-aminobutyric acid; Dha: Dehydroalanine; Dhb: Dehydrobutyrine.