Figure 4.
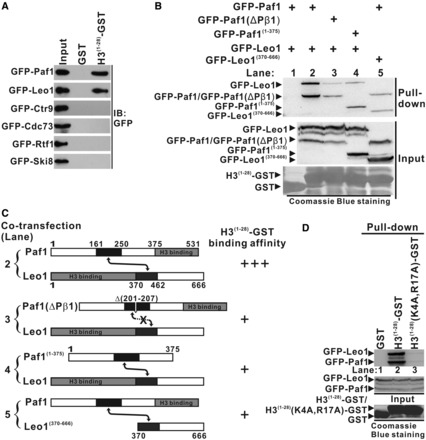
Interaction of the Paf1/Leo1 subcomplex with histone H3. (A) GST pull-down assays of histone H3 with each subunit in PAF1C. H3(1–28)-GST fusion proteins or GST alone were incubated with the extracts of HEK293T cells transfected with each PAF1C subunit. The input lane shows 20% input for the corresponding pull-down. The PVDF membrane was immunoblotted with α-GFP. (B) GST pull-down assays of the Paf1/Leo1 subcomplex with histone H3. H3(1–28)-GST fusion proteins were incubated with the extracts of HEK293T cells transfected with the Pafl/Leo1, the Paf1(ΔPβ1)/Leo1, the Paf1(1–375)/Leo1 and the Paf1/Leo1(370–666) complex, respectively. The PVDF membrane was immunoblotted with α-GFP (top panel) and subsequently stained with Coomassie blue (bottom panel). The middle panel represents 20% of the input material for the corresponding pull-down. (C) Summary of the results obtained in (B). A schematic representation of various combinations of Paf1/Leo1 complex used in the GST pull-down assays is shown. Greater numbers of “+”s correlate with higher H3(1–28)-GST binding affinity with corresponding Paf1/Leo1 complex. (D) GST pull-down assays of the Paf1/Leo1 subcomplex with wild-type and mutant histone H3. H3(1–28)-GST and H3(1–28)(K4A, R17A)-GST fusion proteins were incubated with the extracts of HEK293T cells transfected with the Pafl/Leo1complex. The PVDF membrane was immunoblotted with α-GFP (top panel) and subsequently stained with Coomassie blue (bottom panel). The middle panel represents 20% of the input material for the corresponding pull-down.
