Abstract
Tithonia diversifolia, a member of Compositae native to Central America that produces showy sunflower-like flowers, became an invasive weed in other continents after it was introduced as an ornamental. Little is known about fungal pathogens infecting this plant. Knowledge of its mycobiota is of interest for future biocontrol programmes for T. diversifolia. In Brazil, a cercosporoid hyphomycete was found associated with intense leaf-spotting of this plant. Based on morphological and molecular data it was recognized as representing a new species of Passalora, and the name Passalora stromatica sp.nov. is introduced here for this taxon. This fungus is described and illustrated herein. It is possible that this fungus is playing a role in Brazil in reducing the invasiveness of T. diversifolia as, contrarily to what has been reported for countries in Africa and Asia, it remains mostly as a garden escape or rural plant in Brazil.
Keywords: Anamorphic fungi, cercosporoid fungi, conidial fungi, dematiaceous hyphomycetes, ITS, LSU, wild sunflower
INTRODUCTION
Tithonia diversifolia (wild sunflower) belongs to Compositae, originated in Central America, and has now spread to several tropical and subtropical regions of the world, an indication of a large ecological plasticity (Pérez et al. 2009). It may occur as robust herbaceous stands or have a shrub habit, and be annual or perennial, depending on the environmental conditions of the area. It is well known in Hispanic American countries, where it is commonly called “árbol maravilla”, “girasol mexicano”, “falso girasol”, “crisantemo de Nitobe”, and “quil amargo” (Cairns 1996). In these regions, the plant is used as animal feed and forage (Pérez et al. 2009). It is also used as green manure in some rice and maize growing areas in Asia and Africa (Jama et al. 2000). Nevertheless, the most important use is as an ornamental, because of the abundant showy sunflower-like flowers, which are produced in autumn and winter (Lorenzi 1995). It is likely that, because of its use as an ornamental, T. diversifolia was introduced throughout the tropics and subtropics. In some regions, finding appropriate environmental conditions, and supposedly free from its co-evolved natural competitors and predators, it became a noxious weed. In southern China, where it was planted in villages and along the roadsides for landscaping, it now constitutes a serious threat to natural ecosystems (Yang et al. 2012). Also, in most parts of Nigeria, it is causing serious invasions in agricultural areas, which, according to Chukwuka et al. (2007), are resulting in their abandonment. According to the latter author, this weed probably was introduced into Nigeria through contaminated batches of maize seed imported from Israel. Perhaps invasions are still in the lag phase in Brazil, when a recently introduced species remains at low numbers, but it is also possible that natural enemies are at play. Tithonia diversifolia has never been targeted in weed biocontrol programmes, and little is known about arthropods and pathogens attacking it in its native ranges. The USDA Fungal Database (http://nt.ars-grin.gov/fungaldatabases/) listed 29 geographical records of 12 fungal taxa in association with this host. These included: four cercosporoid fungi (Cercospora tithoniae, C. tithoniicola, Passalora tithoniicola, and Phaeoramularia tithoniae); two rusts (Puccinia enceliae and P. tithoniae); one powdery mildew (Oidium sp.); and two other pathogens (Sclerotinia sclerotiorum, Ulocladium sp.). Additionally, the name Corynespora tithoniae is listed on this host as occurring in China, but that name does not appear in the other main lists of fungal taxa (Index Fungorum and MycoBank), and requires investigation as to its status. Although the host is native to Central America, only two out of the 29 records are from that region, indicating a the lack of knowledge of the fungi occurring on this host.
No fungus has been recorded previously on T. diversifolia in Brazil. Now, severe leaf spotting symptoms have been observed on T. diversifolia at several localities in the highlands of the state of Rio de Janeiro. Only one fungus was found associated with these spots. This work describes this fungus as a new species based on morphological and molecular data.
MATERIAL AND METHODS
Isolates
Samples of diseased leaves of Tithonia diversifolia were collected in the municipality of Nova Friburgo (state of Rio de Janeiro, Brazil), dried in a plant press, and brought to the laboratory for further examination. All samples were examined under a Olympus SZX7 dissecting microscope, and a dematiaceous hyphomycete was found associated with the diseased tissues. Isolation of the fungus into pure culture was performed by the transfer of conidia, using a sterile fine-pointed needle, from lesions onto plates containing VBA (vegetable broth-agar) as described in Pereira et al. (2003). Pure cultures were deposited in the fungal culture collection at the Universidade Federal de Viçosa (UFV) Coleção Octávio Almeida Drummond (COAD). A representative herbarium sample was also deposited in the herbarium at the Universidade Federal de Viçosa (VIC). Slides containing fungal structures were prepared by scraping the structures from colonized tissues with a scalpel or preparing sections with the help of a freezing microtome (Microm HM 520) and then mounting these in lactophenol or lactofuchsin. Observations of fungal structures and measurements, as well as preparation of photographs, were performed with an Olympus BX 51 light microscope fitted with an Olympus E330 camera and a drawing tube. Colony characters and pigment production were noted after 6 d of growth on vegetal brothagar (VBA) and potato carrot agar (PCA) in the incubator at 25 °C and under a 12 h photoperiod. Colony colours (surface and reverse) were rated according to the colour charts of Rayner (1970).
DNA isolation, amplification and analyses
Genomic DNA was isolated from 7-d-old fungal mycelium grown in liquid medium (potato dextrose) in plates. Extraction was performed with Wizard® Genomic DNA Purification Kit (Promega, WI) according to the manufacturer’s instructions. The primers LR0R and LR5 (Vilgalys & Hester 1990) were used to amplify part of the large ribosomal subunit (LSU) and the primers ITS4 and ITS5 (White et al. 1990) were used to amplify the ITS areas as well as the 5.8S rRNA gene. Each 25 μL of PCR reaction consisted of the 2 μL (20 ng) of DNA, 12.5 μL of the Dream Taq Master Mix (Fermentas) and 10 μM of each primer. The LSU PCR was performed as described by Zhang et al. (2009) and the ITS PCR was performed as described by Groenewald et al. (2005). The nucleotide sequence data were obtained by DNA sequencing (Macrogen, Korea) employing the same primers used for PCR amplification. The sequence fragments were assembled from the forward and reverse sequences with the Staden package v. 1.6.0. The ITS and LSU consensus sequences were deposited in GenBank with accession numbers KC841912 and KF275128, respectively.
TAXONOMY
Passalora stromatica A.F. Fernandes & R.W. Barreto, sp. nov.
MycoBank MB804540
Fig. 1.
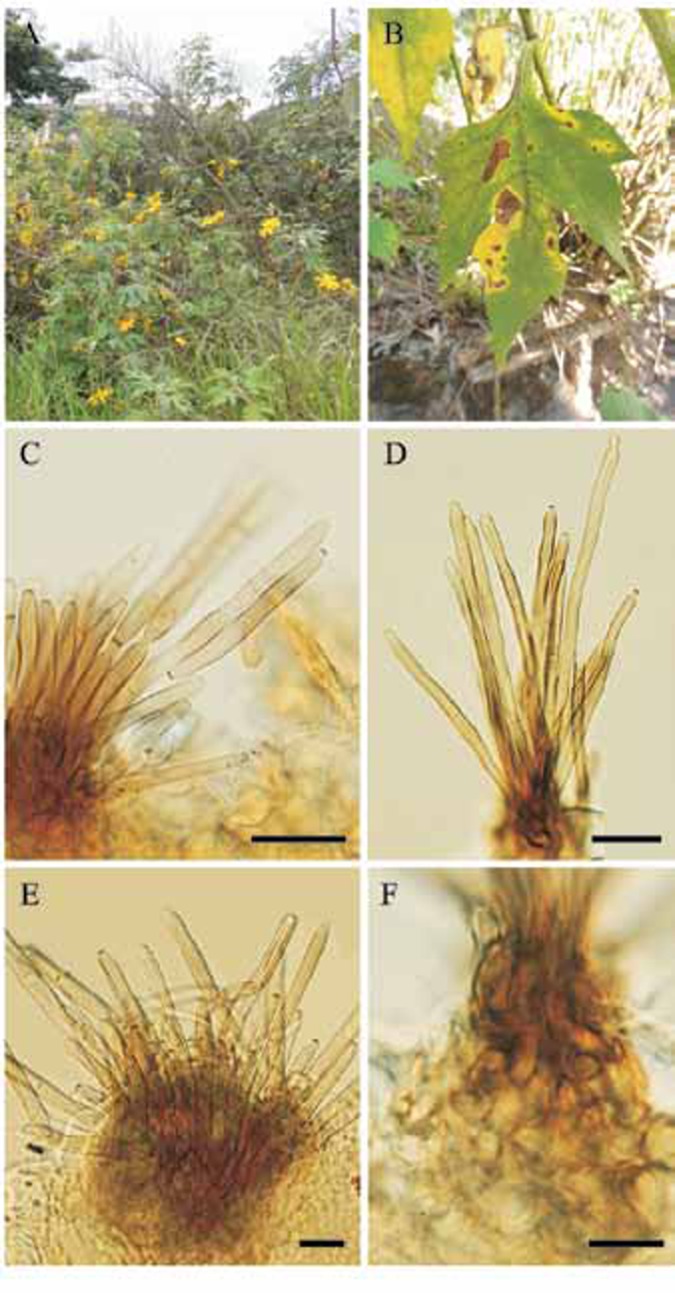
A. Tithonia diversifolia growing in a ruderal situation in the campus of the Universidade Federal de Viçosa (Viçosa, MG, Brazil). B. Leaf spots on T. diversifolia. C–F. Passalora stromatica (VIC 31925). C. Conidiophores and conidia. D. Conidiophores of P. stromatica. E. Conidiophores and stroma. F. Stroma. Bars = 15 μm.
Fig. 2.
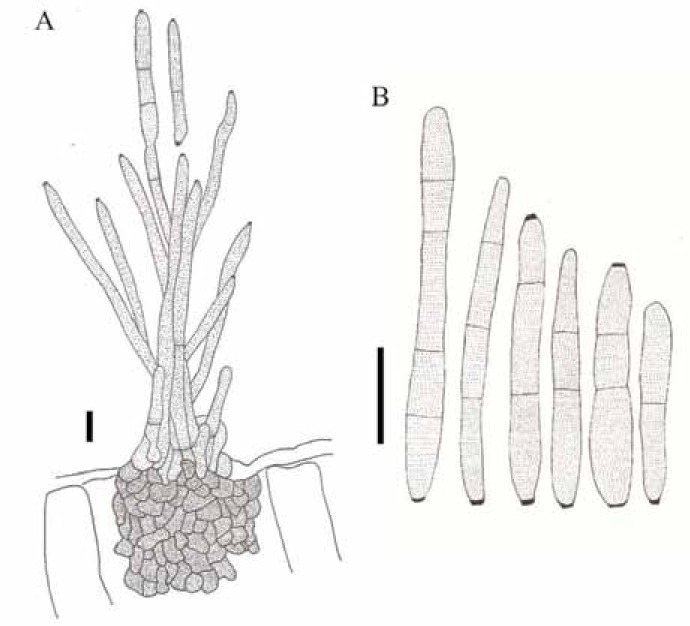
Passalora stromatica (VIC 31925). A. Conidiophores arising from a stroma and conidia. B. Conidia. Bars = 15 μm.
Etymology: Based on the well-developed stromata formed by the fungus.
Diagnosis: Differs from Passalora tithoniae in having much larger stromata, 12.5–70 × 15–55 μm, and broader conidia, 2.5–6.5 μm wide.
Type: Brazil: Rio de Janeiro: Nova Friburgo, Conquista on leaves of Tithonia diversifolia, 9 July 2011, R. W. Barreto (VIC 31925 – holotype; culture ex-type COAD 1099; GenBank: ITS= KF275128, LSU = KC841912).
Description: Lesions on living leaves starting as small necrotic dots, becoming angular to irregular with well delimited edges, dark brown with a greyish white centre, 7–59 mm diam, surrounded by a large irregular area of yellowing tissue; when the disease progresses, lesions coalescing, leading to the development of leaf blight areas and defoliation. Internal mycelium intra- and intercellular, hyphae branched, septate, 3–6 μm wide, brown. External mycelium absent. Stromata subepidermal, irregular, 12.5–70 × 15–55 μm, composed of dark brown textura angularis. Conidiophores amphigenous, densely fasciculate, cylindrical, 48.5–174 × 4–8 μm, 0–4-septate, dark brown at the base, lighter towards the apex, smooth. Conidiogenous cells holoblastic proliferating sympodially, terminal and intercalary, integrated, cylindrical, 9–38.5 × 4–8 μm, pale brown; conidiogenous loci (scars) conspicuous, 2.5–4 μm diam, darkened and thickened. Conidia solitary or in simple chains, subcylindrical to obclavate, 15.5–61.5 × 2.5–6.5 μm, pale brown, smooth, rounded at the apex, the base obconic, 1.5–3 μm diam at the base, 0–2-septate, hila 1–2 per conidium, darkened and thickened.
Culture characteristics: Colonies slow-growing, 13–27 mm diam after 30 d incubated at 25 °C, on VBA, olivaceous grey, felty and raised centrally, becoming powdery and flat towards the periphery, margin slightly lobed, with strong diurnal zonation; on PCA, colony irregular with a bird dropping appearance, white and cottony in centre, woolly and iron-grey at periphery; fertile.
DISCUSSION
The fungus on Tithonia diversifolia is a typical cercosporoid fungus, having conidia and conidiophores that are pigmented and bearing slightly thickened and darkened conidiogenous loci. This combination of morphological features places it in the genus Passalora as accepted by Crous & Braun (2003). Homology search with ITS and LSU rDNA sequences using a BLAST search on the GenBank nucleotide database also confirmed a placement within Passalora. The closest matches using the LSU sequence were two unnamed Passalora species with 99 % of similarity (GenBank accession numbers GU214668 and GQ852623). The closest hits using the ITS sequence were also two Passalora species with 95 % of homology (GenBank accession numbers GU214668 and GU214639). Four cercosporoid taxa have been reported on members of the genus Tithonia. Passalora stromatica, however, is well characterized by the very broad conidiophores, grouped in loose fascicles on a well-developed stroma. There is another species of Passalora described on T. diversifolia, P. tithoniae, but that species has poorly developed stromata, and narrower conidiophores (3–4 μm vs. 4–8 μm; Crous & Braun 2003) and is, hence, clearly distinct. It is therefore necessary to describe P. stromatica for the fungus found on T. diversifolia in Brazil.
The impact of this fungus on T. diversifolia makes it a potential good candidate for the biocontrol of this weed in Asia and Africa. However, further studies are necessary to ascertain its host-specificity.
Acknowledgments
Funding from the Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) is acknowledged.
REFERENCES
- Cairns MF. (1996) Study on Farmer Management of Wild Sunflowers (Tithonia diversifolia). Bogor: International Centre for Research in Agroforestry S E. Asian Regional Research Programme [Google Scholar]
- Chukwuka KS, Ogunyemi S, Fawole I. (2007) Ecological distribution of Tithonia diversifolia (Hemsl.) A. Gray – a new exotic weed in Nigeria. Journal of Biological Science 7: 709–719 [Google Scholar]
- Crous PW, Braun U. (2003) Mycosphaerella and its anamorphs. 1. Names published in Cercospora and Passalora. [CBS Biodiversity Series no. 1.] Utrecht: KNAW/CBS Fungal Biodiversity Centre [Google Scholar]
- Groenewald M, Groenewald JZ, Crous PW. (2005) Distinct species exist within the Cercospora apii morphotype. Phytopathology 95: 951–959 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2001) MrBayes: Bayesian inference of phylogeny. Bioinformatics 17: 754–755 [DOI] [PubMed] [Google Scholar]
- Jama B, Palm CA, Buresh RJ, Niang A, Gachengo C, Nziguheba G, Amadalo B. (2000) Tithonia diversifolia as a green manure for soil fertility improvement in western Kenya: a review. Agroforestry Systems 49: 201–221 [Google Scholar]
- Lorenzi H. (1995) Plantas Ornamentais no Brasil: arbustivas, herbáceas e trepadeiras. Nova Odessa, SP: Editora Plantarum [Google Scholar]
- Pereira JM, Barreto RW, Ellison C, Maffia LA. (2003) Corynespora cassiicola f. sp. lantanae: a potential biocontrol agent for Lantana camara from Brazil. Biological Control 26: 21–31 [Google Scholar]
- Pérez A, Montejo I, Iglesias JM, López O, Martín GJ, García DE, Milián I, Hernández A. (2009) Tithonia diversifolia (Hemsl.) A. Grey. Pastos y Forrajes 32: 1–15 [Google Scholar]
- Rayner RW. (1970) A Mycological Colour Chart. Kew: Commonwealth Mycological Institute [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds): 315–322 San Diego: Academic Press [Google Scholar]
- Yang J, Tang L, Guan Y, Sun W. (2012) Genetic diversity of an alien invasive plant Mexican sunflower (Tithonia diversifolia) in China. Weed Science 60: 552–557 [Google Scholar]
- Zhang Y, Schoch CL, Founier J, Crous PW, de Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Spatafora JW, Hyde KD. (2009) Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology 64: 85–102 [DOI] [PMC free article] [PubMed] [Google Scholar]