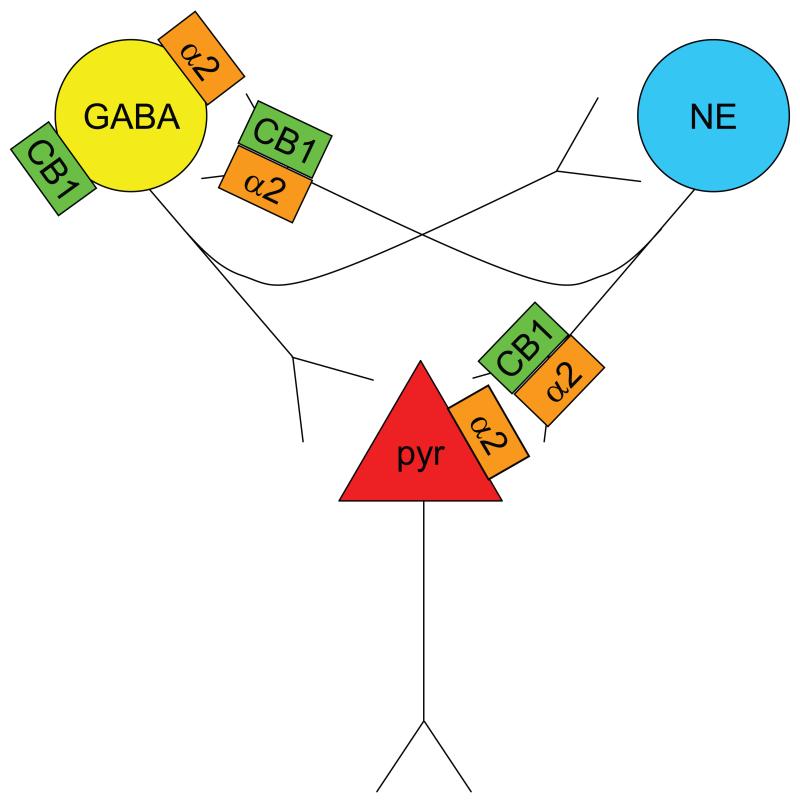
Figure 8.
Schematic diagram showing putative mechanisms underlying cannabinoid modulation of norepinephrine. Microdialysis and electrophysiology data indicate cannabinoid-induced de-sensitization of pre- and post-synaptic α2-ARs, respectively. Presynaptic α2-AR de-sensitization will lead to cannabinoid-induced increases in cortical NE efflux. Electrophysiological studies demonstrate blockade of α2-AR-mediated pyramidal cell excitability by WIN implicating de-sensitization of postsynaptic α2-ARs in the effect. Stress is impacting CB1-α2-AR interactions at both pre- and post-synaptic levels. In summary, the microdialysis study suggests that cannabinoids restrain stress-induced NE efflux. The electrophysiological studies suggest that cannabinoids restrain PFC cell excitability via de-sensitization of postsynaptic α2-ARs but stress can interfere with this interaction, potentially contributing to over-activation of pyramidal neurons in PFC. Alternatively, cannabinoids may desensitize α2-AR on GABA interneurons, resulting in increased GABA tone and potential restraint of both cortical excitability and NE efflux. If stress interferes with this desensitization, the effect would be decreased GABA tone, excess NE release and excitability of cortical neurons. Future studies are required to address local network interactions. Overall, cannabinoids are protective of the NE system and cortical excitability but stress can derail this protective effect, leading to psychopathology. Abbreviations: Acb-nucleus accumbens; α2-AR-α2-adrenergic receptor; aCSF-artificial cerebrospinal fluid; CB1-cannabinoid receptor 1; GABA-gamma amino butyric acid; HPLC-ED-high performance liquid chromatography with electrochemical detection; LC-locus coeruleus; mPFC-medial prefrontal cortex; NE-norepinephrine; PTSD-post-traumatic stress disorder; pyr-pyramidal neurons; TH-tyrosine hydroxylase;.