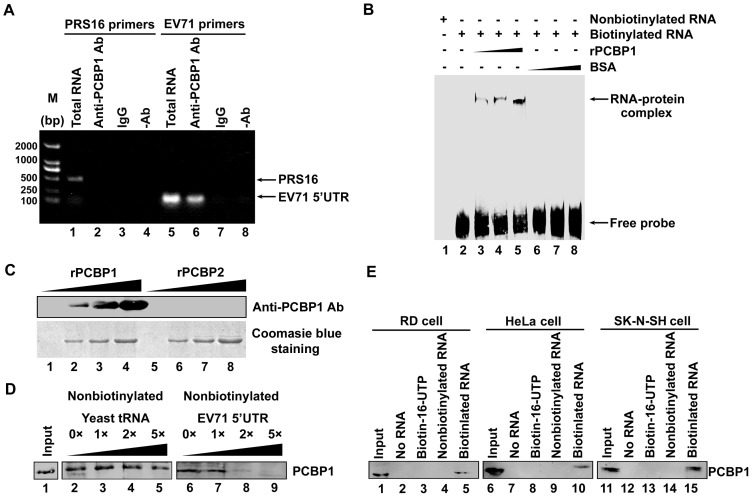
Figure 1. Interaction between PCBP1 protein and the 5′UTR of EV71 RNA.
(A) Co-immunoprecipitation (Co-IP) and RT-PCR assay. RD cells were infected with EV71 at an MOI of 40 for 6 h and then cell extracts were prepared. For Co-IP assay, extract of EV71-infected cells was incubated with mouse anti-PCBP1 antibody (lanes 2 and 6), normal mouse IgG (lanes 3 and 7), or without antibody (-Ab) (lanes 4 and 8). Following washing and dissociation, the RNA extract was prepared and subjected to RT-PCR analysis with primers specific for the ribosomal protein S16 (PRS16) RNA (lanes 1–4) or for EV71 5′UTR RNA (lanes 5–8). Total RNA from cell extracts without Co-IP was evaluated by RT-PCR as a control (lanes 1 and 5). RT-PCR products were separated and detected by agarose gel electrophoresis and the expected band is indicated by an arrow. (B) Electrophoretic mobility shift assay (EMSA). Plasmid carrying EV71 5′UTR DNA was linearized and in vitro transcribed into RNA, which was then labeled with biotin-16-UTP at the 3′ end. Labeled RNA (0.5 µg) was incubated with recombinant PCBP1 (rPCBP1) at 0, 0.1, 0.15 and 0.2 µg (lanes 2 to 5) or BSA at 0.1, 0.15, and 0.2 µg (lanes 6 to 8), respectively. The RNA-protein complex (indicated by an arrow) was separated from free probe RNA and examined by EMSA. No-labeled RNA was in lane 1 as a control. (C) The specificity of the PCBP1 antibody. Purified rPCBP1 protein (lanes 1 to 4) and rPCBP2 protein (lanes 5 to 8) were subjected to SDS-PAGE at 0, 2, 4 and 8 µg, respectively, stained by Coomasie blue and then detected by specific PCBP1 antibody. (D) Biotinylated RNA/protein pull-down assays. For RNA/protein pull-down assay and for competition assay, cell extracts were mixed with biotinylated EV71 5′UTR RNA (lanes 2–9) along with different concentrations of nonbiotinylated yeast tRNA (lanes 2–5) or nonbiotinylated EV71 5′UTR RNA (lanes 6–9), respectively. After pull-down assay, the bound proteins were boiled and subjected to 12% SDS-PAGE and analyzed by western blotting with a mouse anti-PCBP1 antibody. The input contained 200 µg cell lysate (lane 1). (E) Extracts of RD cells (lanes 1–5), HeLa cells (lanes 6–10), and SK-N-SH cells (lanes 11–15) were prepared and then incubated without RNA (lanes 2, 7, and 12), with biotin-16-UTP (lanes 3, 8, and 13), non-biotinylated EV71 5′UTR (lanes 4, 9, and 14), or biotinylated EV71 5′UTR (lanes 5, 10, and 15). After pull-down assay, the bound proteins were boiled, eluted, and subjected to 12% SDS-PAGE and PCBP1 protein was detected by western blot with a mouse anti-PCBP1 antibody. The inputs were cell extracts of RD (lane 1), HeLa (lane 6), and SK-N-SH (lane 11).