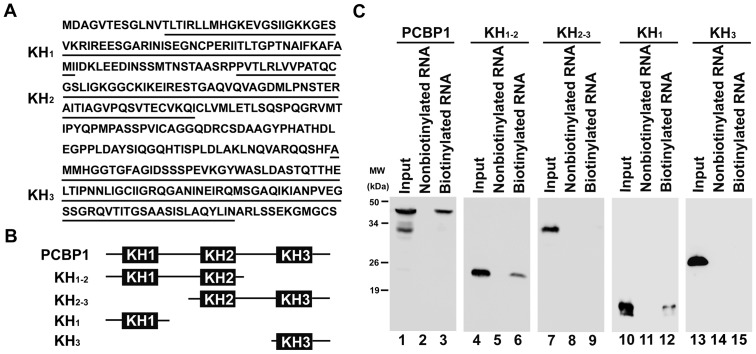
Figure 3. Analysis of the function domain of PCBP1 protein required for its binding to the 5′UTR of EV71 RNA.
(A) The KH domains of full-length PCBP1 protein were predicted by PROSITE tools (http://prosite.expasy.org/). The amino acids of the three KH domains, KH1, KH2, and KH3, were underlined. (B) Based on the sequences of PCBP1 protein and its predicted domains, five plasmids expressing the full-length PCBP1 or truncated PCBP1 proteins were constructed, in which the KH3 was deleted (KH1-2), the KH1 was deleted (KH2-3), both KH2 and KH3 were deleted (KH1), or both KH1 and KH2 were deleted (KH3), respectively. (C) For the RNA-protein binding assay, HeLa cells were transfected with plasmids coding the Flag-tagged full-length PCBP1 (lanes 1-3) or the Flag-tagged truncated proteins, PCBP1-KH1-2 (lanes 4–6), PCBP1-KH2-3 (lanes 7–9), PCBP1-KH1 (lanes 10–12), and PCBP1-KH3 (lanes 13–15), respectively. For the pull-down assay, cell extracts were prepared and mixed with nonbiotinylated EV71 5′UTR RNA (lanes 2, 5, 8, 11, and 14) or biotinylated EV71 5′UTR RNA (lanes 3, 6, 9, 12, and 15), respectively. The complexes were boiled and dissolved and PCBP1 protein was determined by western blot analysis using anti-Flag antibody (lanes 2, 3, 5, 6, 8, 9, 11, 12, 14, and 15). The inputs were cell extracts without pull-down assay (lanes 1, 4, 7, 10, and 13).