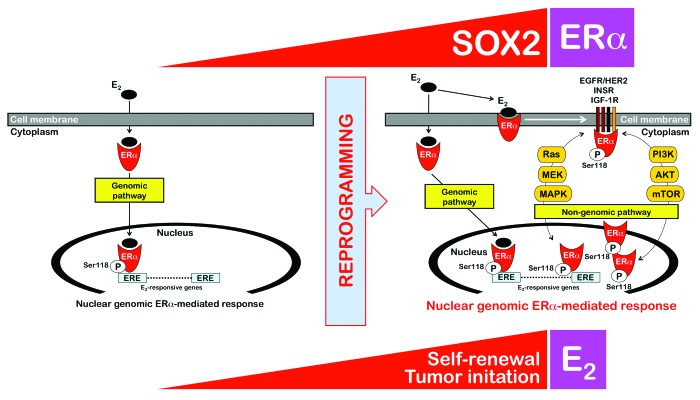
Figure 4. Reprogramming of non-genomic estrogen signaling by SOX2: A new driver of breast cancer stem-like cellular states with tumor-initiating capacity. SOX2 overexpression confers a tumor-initiating advantage to ERα-positive MCF-7 breast cancer cells in an estrogen-dependent manner, i.e. when s.c. injected into ovariectomized nude mice implanted with estrogen pellets, SOX2-overexpressing MCF-7/Rep cells formed more and larger tumors than MCF-7 parental cells, which mostly failed to produce tumor outgrowths in the absence of Matrigel. The previously unrecognized ability of the stemness factor SOX2 to promote estrogen-dependent tumorigenesis of ERα-positive breast cancer cells appears to depend on the activation of non-genomic estrogen signaling. E2 activates nuclear ERα (genomic pathway) and ERα in or near the membrane (non-genomic pathway). Membrane-associated ERα associates with growth factor signaling components (e.g., EGFR/HER2, INSR, IGF-1R), thus allowing E2 to activate the growth factor signaling by activating key molecules such as PI3K or Ras and downstream molecules such as AKT, mTOR, MEK, and MAPK. The crosstalk between the non-genomic estrogen signaling and the growth factor signaling is bidirectional, because a variety of kinases including MAPKs, AKT, and mTOR coordinately regulate the phosphorylation of specific sites of the ERα, leading to ligand (E2)-independent ERα activation. Moreover, phosphorylation of ERα co-regulatory proteins by growth factor kinases regulates the ERα signaling pathway to enhance the nuclear genomic ERα-mediated response. Our findings strongly suggest that reprogramming of non-genomic estrogen signaling by the stemness factor SOX2 is a key molecular feature that determines the tumor-initiating capacity of breast cancer stem-like cellular states.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.