Abstract
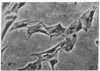
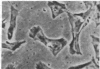
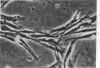
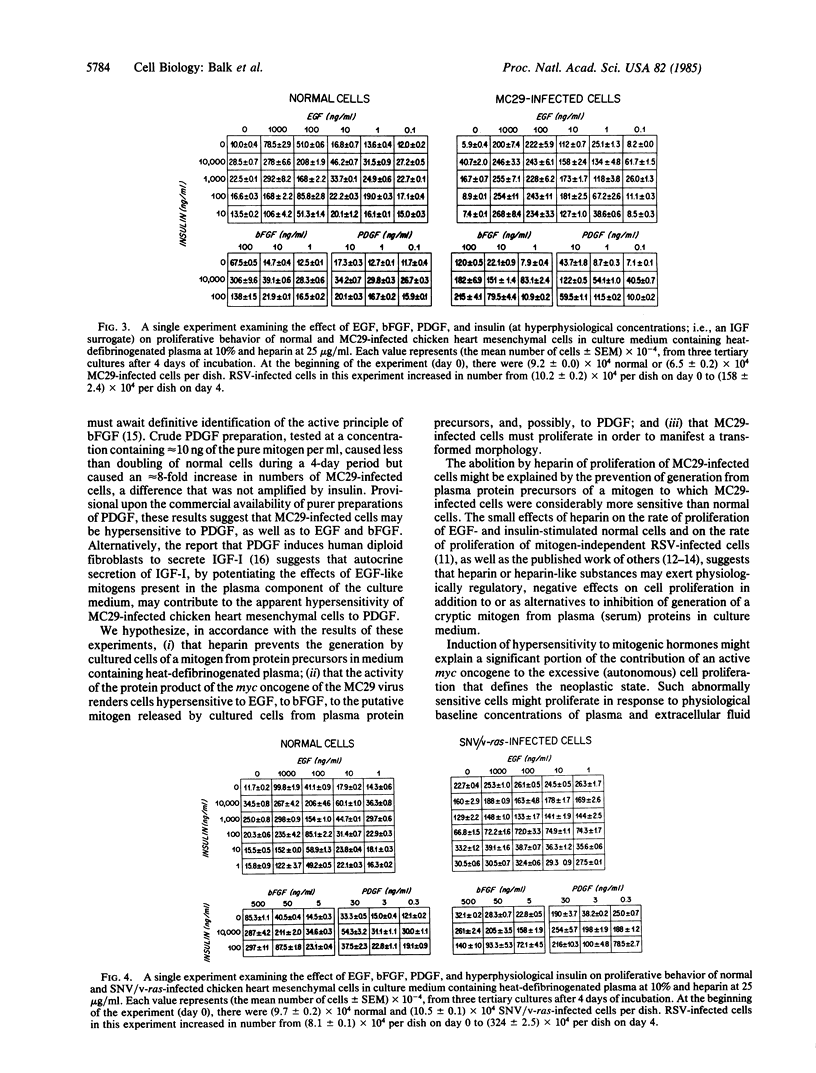
Chicken heart mesenchymal cells do not proliferate in culture medium containing heat-defibrinogenated plasma but proliferate briskly when incubated with epidermal growth factor (EGF) or brain fibroblast growth factor (bFGF) plus insulin-like growth factors (IGFs) or when infected with sarcoma or erythroblastosis viruses. When infected with the retrovirus MC29, which bears a v-myc oncogene, chicken heart mesenchymal cells proliferate at a more modest rate and become morphologically transformed. Heparin at 25 microgram/ml causes these MC29-transformed cells to become proliferatively quiescent and to assume a normal morphology. Heparin-treated MC29-infected cells are, however, 100 times more sensitive to EGF than are their normal, uninfected counterparts. MC29-infected cells appear, likewise, to be hypersensitive to bFGF and to PDGF preparations but not to insulin. We hypothesize, therefore, (i) that heparin prevents the generation by cells of a mitogen from plasma protein precursors in the culture medium; (ii) that the v-myc oncogene renders cells hypersensitive to EGF, bFGF, PDGF, and the putative plasma-protein-derived mitogen; and (iii) that MC29-infected cells must proliferate in order to manifest the transformed morphology. Chicken heart mesenchymal cells infected with a recombinant spleen necrosis virus containing a v-ras oncogene are morphologically transformed but proliferate only sluggishly in plasma-containing medium without added mitogenic hormones. Heparin does not significantly affect their behavior. They are refractory to mitogenic stimulation by EGF or bFGF suggesting that ras proteins mediate the effects of receptors for these hormones. The SNV/v-ras-infected cells proliferate briskly, however, in response to hyperphysiological concentrations of insulin, an IGF surrogate, and are considerably more sensitive to this IGF mitogenicity than are their normal, uninfected counterparts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Balk S. D. Active proliferation of Rous sarcoma virus-infected, but not normal, chicken heart mesenchymal cells in culture medium of physiological composition. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6606–6610. doi: 10.1073/pnas.77.11.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D., Gunther H. S., Morisi A. Morphological transformation, autonomous proliferation and colony formation by chicken heart mesenchymal cells infected with avian sarcoma, erythroblastosis and myelocytomatosis viruses. Life Sci. 1984 Sep 10;35(11):1157–1171. doi: 10.1016/0024-3205(84)90186-3. [DOI] [PubMed] [Google Scholar]
- Balk S. D., Levine S. P., Young L. L., LaFleur M. M., Raymond N. M. Mitogenic factors present in serum but not in plasma. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5656–5660. doi: 10.1073/pnas.78.9.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D., Morisi A., Gunther H. S. Phorbol 12-myristate 13-acetate, ionomycin or ouabain, and raised extracellular magnesium induce proliferation of chicken heart mesenchymal cells. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6418–6421. doi: 10.1073/pnas.81.20.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D., Morisi A., Gunther H. S., Svoboda M. F., Van Wyk J. J., Nissley S. P., Scanes C. G. Somatomedins (insulin-like growth factors), but not growth hormone, are mitogenic for chicken heart mesenchymal cells and act synergistically with epidermal growth factor and brain fibroblast growth factor. Life Sci. 1984 Jul 23;35(4):335–346. doi: 10.1016/0024-3205(84)90643-x. [DOI] [PubMed] [Google Scholar]
- Balk S. D., Shiu R. P., LaFleur M. M., Young L. L. Epidermal growth factor and insulin cause normal chicken heart mesenchymal cells to proliferate like their Rous sarcoma virus-infected counterparts. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1154–1157. doi: 10.1073/pnas.79.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Beeler D. L., Rosenberg R. D., Karnovsky M. J. Structural determinants of the capacity of heparin to inhibit the proliferation of vascular smooth muscle cells. J Cell Physiol. 1984 Sep;120(3):315–320. doi: 10.1002/jcp.1041200309. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Underwood L. E., Van Wyk J. J. Hormonal control of immunoreactive somatomedin production by cultured human fibroblasts. J Clin Invest. 1981 Jan;67(1):10–19. doi: 10.1172/JCI110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Fritze L. M., Reilly C. F., Rosenberg R. D. An antiproliferative heparan sulfate species produced by postconfluent smooth muscle cells. J Cell Biol. 1985 Apr;100(4):1041–1049. doi: 10.1083/jcb.100.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]