Abstract
Raising high titer antibodies in animals is usually performed by protein immunization, which requires the long and sometimes difficult step of production of the recombinant protein. DNA immunization is an alternative to recombinant proteins, only requiring the building of an eukaryotic expression plasmid. Thanks to efficient DNA delivery techniques such as in vivo electroporation, DNA vaccination has proven useful the last few years. In this work, we have shown that it is possible to raise very high antibody titers in rabbit by DNA electroporation of an antigen encoding plasmid in the skeletal muscle with the right set of electrodes and rabbit strain. In a model of botulinum toxins types A and E, the neutralizing titers obtained after three treatments were high enough to fit the European Pharmacopeia, while it did not for type B toxin. Furthermore, the raised antibodies have high avidity and are suitable for in vitro and in vivo immunodetection of proteins.
Keywords: DNA immunization, electroporation, botulinum neurotoxin, antiserum, neutralizing antibodies
Introduction
The botulinum neurotoxins (BoNTs) have been characterized to be the most potent toxic substances identified so far. They have been accounted for several food poisoning in human and animal cases.1,2 Among the seven serologically distinct types of BoNTs (A to G), BoNTs type A, B, E and F are commonly linked to human disease, BoNT/A being the deadliest of all with an estimated lethal dose of a nanogram per kilogram of body weight.1,3
Botulinum neurotoxin consists of a poorly active, single polypeptide chain of 150kDa, which is posttranslationnaly cleaved to an active disulfide-linked double-chain. This double chain comprises of a light subunit (LC, about 50 kDa) and a heavy subunit (HC, about 100 kDa). The toxin is composed of three functional domains.4 The C-terminal half of HC (fragment C, noted Fc) mediates the binding to the target neurons, which triggers the internalization of the whole toxin into endocytic vesicles. The N-terminal half of HC mediates the translocation of LC, which is the intracellular active domain into the cytoplasm of the neuron. In motor nerve endings and autonomic cholinergic junctions, BoNTs cleave one of the three proteins SNARE (Soluble NSF Attachment Protein Receptor), synaptobrevin, SNAP-25, or syntaxin, which constitute the synaptic fusion complex and have a determinant role in neuroexocytosis. Thus, BoNTs block the release of acetylcholine leading to flaccid paralysis.5
Botulism is naturally a relatively rare disease in humans. However, based on their high toxicity, BoNTs are considered as potential biological weapons via aerosols, which could raise the necessity to develop a vaccine against these toxins. Furthermore, BoNTs are currently used as FDA approved therapeutic agents for the treatment of numerous diseases such as dystonias or strabismus, or for cosmetic surgery.6 Moreover, multiple novel applications (non FDA approved) are currently being used for the treatment of various disorders in a variety of medical fields. Because of these implications, the use of toxoid vaccine may not be suitable and thus, better strategies to neutralize botulinum toxins, including the production of safe and effective anti-BoNT antisera are in need to be devised.
There is currently no cure for botulism after the beginning of the symptoms. Although some efficient neutralizing monoclonal antibodies start to be studied at the laboratory level,7 current therapies for botulism consist mainly of supportive care, active vaccination and passive immunization with antiBoNT antisera which are supposed to eliminate the circulating toxin before binding to the neuromuscular junction, thus reducing the duration and the severity of the disease. The most clinically used antitoxin antibodies are the botulinum immunoglobulin BabyBIG which is a human polyclonal antibody of very limited supply used to treat infants,8 and an equine polyclonal antisera.1 Different strategies have been used for inducing immune response against botulinum toxin (see ref. 9 for a review). Neutralizing antisera are generally raised after animal immunization with formalin-detoxified toxins or more recently recombinant or chemically-altered derivatives of the toxins,10-13 which requires recombinant protein purification or handling of the full toxins. Genetic immunization is an effective alternative to recombinant proteins immunization to raise an efficient immune response against an antigen. Actually, genetic immunization through an adenoviral vector led protective immunity in mice against botulinum toxin type C.14
Particularly, genetic immunization by the non-viral intramuscular DNA electroporation technique is cost-effective and is widely used in the fields of infectious diseases vaccination or cancer immunotherapy, see refs. 15–17 for reviews. It involves the application of electric pulses after intramuscular injection of an antigen-coding plasmid DNA, which enhances plasmid cellular uptake and hence the immunogenicity and vaccine efficiency. This technique requires only plasmid DNA, which can be easily produced under good manufacturing production conditions. Furthermore, intramuscular electroporation leads to sustained protein production in muscles for more than several months, and secretion into the blood circulation. Thus, long-lasting antibody production is expected in the treated animals. We have previously shown that it was possible to raise high titer neutralizing antibodies against botulinum toxins A, B and E in mice using this technique and plasmids containing codon-optimized sequences of the non-toxic Fc fragments of these toxins.18 However, efficiency in larger animals is required if one wants to produce large amounts of antibodies. We have previously shown that DNA electroporation in rabbits may lead to functional antibodies against malaria antigens.19 In this article, we investigated the possibility of raising high titer antiserum against botulinum toxins A, B and E in rabbits using in vivo intramuscular DNA electroporation technique. Rabbit may be used to produce physiologically active polyclonal antibodies, as attested by the clinically used Thymoglobulin (Genzyme). Thymoglobulin (antithymocyte globulin) is a rabbit polyclonal anti–T-cell antibody preparation obtained by immunization with human thymocytes. Thymoglobulin is widely used for the treatment of rejection after organ transplantation.20 In the present work, after the set-up of the optimal immunization procedure in mice, we show that DNA immunization by electroporation in rabbits leads to high-titer antiserum against botulinum toxins A and E, but not B. Furthermore, the raised antibodies have proven useful for immunodetection both in vitro in transfected cells and in vivo in skeletal muscle sections. This is to our knowledge the first report of very high neutralizing antiserum production in rabbit after DNA electroporation. DNA electroporation might then be a viable alternative to classical protein immunization for antibody production, not only against botulinum toxins, but for a wide range of antigens.
Results
Improvement of immunization protocol in mice
Three different plasmids were used in the present work and encode respectively the Fc C-terminal heavy chain fragment of neurotoxins A, B and E (plasmids pVax-FcBoNT/A), pVax-FcBoNT/B and pVax-FcBoNT/E). These coding sequences have been codon-optimized to fit the mouse usage of the genetic code and fused to the mouse erythropoietin secretion signal as described in ref. 18. In this previous study, we have shown that immunizing mice by electroporating these DNA plasmids into the skeletal tibialis anterior skeletal muscle led to generation of high titer total antibodies in mice against botulinum toxins types A, B and E. The best neutralizing titer against type A toxin was 20 international units/ml (IU/ml) after two electroporation treatments, knowing that 1 IU corresponds to neutralization of 5,000 mouse lethal dose (MLD). To investigate whether a better protocol could be set up to generate higher neutralizing antiserum or not, the antibody induction in mice was measured after different immunization regimens. Groups of 4 mice were immunized with 40 µg of pVax-FcBoNT/A plasmid at day 0, 30, and 60 if required, and the antibody production was measured 15 d after the second and third immunization. All plasmid DNA electroporation in mice were performed using the electrical conditions usually used in our group and already described in ref. 18, i.e., eight square wave electric pulses of 200 V/cm and 20 ms duration at a frequency of 5 Hz, using plate electrodes. Neutralizing titers were determined by mouse protection assay at day 75. Results are shown in Figure 1. As already shown in ref. 18, animals treated with two electroporation of plasmid led to a neutralizing titer of 20 IU/ml. When a third immunization boost was performed, the total antibody titer increased from 2,800 to 5,500 if this third treatment was a DNA plasmid electroporation boost, and to 10,900 if it was a recombinant FcBoNT/A protein boost (1 µg in alum, intraperitoneally). More importantly, by using a third immunization boost whether DNA or protein, the neutralizing titers against botulinum toxin A at day 75 dramatically increased from 20 IU/ml to 100 IU/ml. Thus, although the total antibody titer was higher with a recombinant protein boost, this did not improve the neutralizing titer compared with a 3 DNA plasmid electroporation treatment. For comparison, a classical immunization with the recombinant FcBoNT/A protein (3 immunizations at 30 d interval) led to the highest total antibody titer (18 000), but no significant improvement of the neutralizing titer was observed (100 IU/ml). A fourth treatment at day 90 either by DNA electroporation or recombinant protein boost did not improve the total and neutralizing antibody titers (data not shown).
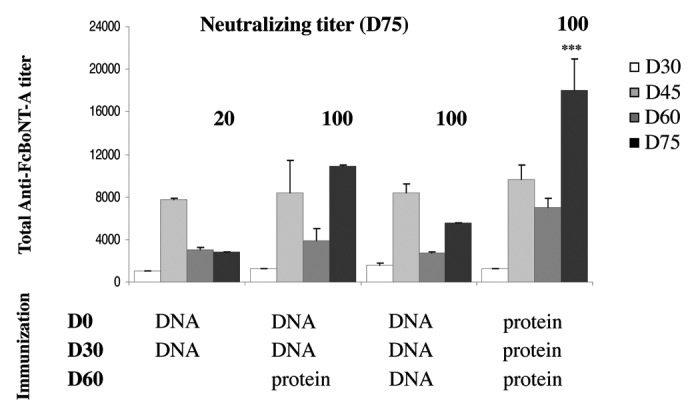
Figure 1. Antibody responses of mice injected with pVax-FcBoNT/A with subsequent electroporation, or recombinant protein A, at day 0 and boosted with plasmid pVax-FcBoNT/A with electroporation or a recombinant protein injection at day 30 and day 60.The immunization regimen are indicated below the figure in the immunization section and the days of treatment appear in bold characters. DNA means a pVax-FcBoNT/A electroporation treatment and protein means a recombinant protein immunization. Swiss mice (n = 4) were treated with 40µg of plasmid DNA or 1µg of recombinant FcBoNT/A homogenized in 1mg aluminum hydroxide. Total antibody titer was determined by ELISA of serum samples at different time points of the treatment at days 30, 45, 60, and 75 and represented as histograms. Results show mean ± SEM values. ***(p < 0.001) indicates a significant difference between total antibody titers obtained with protein injections and those obtained with plasmid electroporation. The neutralizing titers at day 75 are indicated in bold characters above the bars for each immunization regimen. Swiss mice (n = 5) were challenged with 10 MLD of BoNT/A mixed with serial dilutions of antiserum collected at day 75. The final neutralizing titer was determined by a mathematical formula (barycenter calculation) using the two highest dilutions, knowing that 1 IU corresponds to neutralization of 5000 MLD.
Upscale of immunization protocol in rabbits
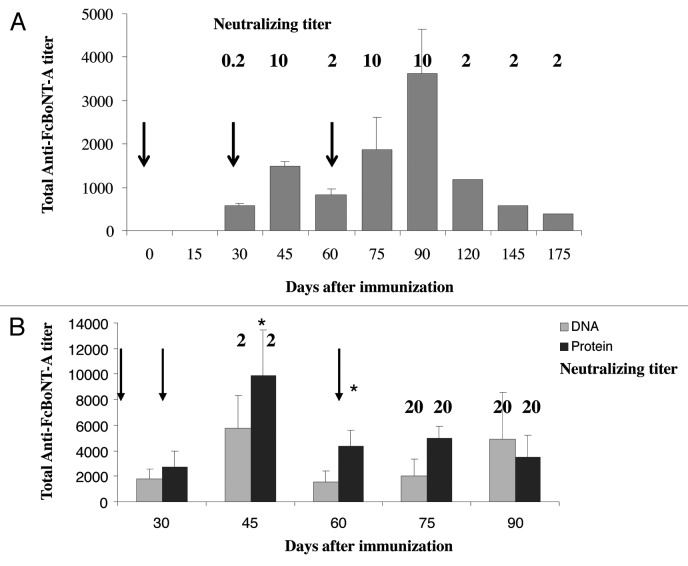
We then investigated if the DNA electroporation technique could be scaled-up to rabbits for induction of neutralizing antibodies against botulinum toxin type A. Experiments were performed at first on New-Zealand rabbits from Hypharm/Groupe Grimaud, with 2-needle electrodes from the BTX Company. As the electric field pattern varies upon the tissue and the electrode type, resulting in a varying effective intensity of the field (in V/cm) in the treated area, we also had to set up efficient conditions for plasmid DNA electroporation in the skeletal muscle of rabbit with two needle electrodes. Unpublished preliminary studies allowed us to set up these electroporation conditions to be eight square wave electric pulses of 120 V/cm and 20 ms duration at a frequency of 5 Hz. These conditions were then used for all rabbit immunizations. More preliminary experiments on different rabbit muscles showed that the longissimus dorsi muscle and the thigh muscle led to the highest immune response (data not shown). Two rabbits (one male, one female) were treated by 4 pVax-FcBoNT/A plasmid electroporations in each lumbar muscle at days 0, 30, and 60. Blood samples were collected by central artery ear bleed, and total antibody titer and toxin neutralizing titer were followed over time for about 6 mo, starting at day 30 (Fig. 2A). Total antibody titers against botulinum toxin A rose after each electroporation treatment, reaching a maximum of 3,500 one month after the third treatment, then decreased over time but remained detectable at day 175. Neutralizing titer was about 0.2 IU/ml one month after the first treatment, then reached 10 IU/ml 15 d after the second treatment, and decreased to 2 IU/ml one month after this treatment. After the third electroporation treatment, the neutralizing titer reached again 10 IU/ml at days 75 and 90, and then decreased to a stable level of 2 IU/ml for the three following months. Increasing the DNA plasmid dose to 1.5 mg per treatment in two other rabbits (one male, one female, Fig. 2B) in the same conditions led to an increase in total antibody titer at day 45, and an increased up to 20 IU/ml in the neutralizing titer at days 75 and 90, where the experiment was terminated. A fourth electroporation treatment did not improve either the total antibody titer or the neutralizing titer. For comparison, one male and one female were also immunized intramuscularly with 400 µg of the FcBoNT/A recombinant protein emulsified in Freund's complete adjuvant for the initial dose at day 0 and in Freund's incomplete adjuvant for the day 30 and day 60 boosts. Although the total antibody titer was higher at day 45 compared with the electroporation treatments (9800 vs. 5800, p < 0.05), the neutralizing titers at day 75 and 90 were also 20 IU/ml.
Figure 2. (A) Antibody responses of rabbits injected with plasmid pVax-FcBoNT/A with electroporation using BTX electrodes at days 0, 30 and 60 as indicated by the arrows. New Zealand white rabbits (n = 2) were treated with 600µg of plasmid pVax-FcBoNT/A by four electroporation treatments in each lumbar muscle (100 µl per site). Total antibody titers were determined by ELISA of serum samples at the indicated different time points of the treatment (days 30, 45, 60, 75, 90, 120, 145, and 175) and represented as histograms. Results show mean ± SEM values. The neutralizing titers at the different time points are indicated in bold characters above the corresponding histogram. (B) Antibody responses of rabbits treated with plasmid pVax-FcBoNT/A with electroporation using the BTX electrodes or recombinant protein FcBoNT/A respectively at days 0, 30 and 60 as indicated by the arrows. New Zealand white rabbits (n = 4) were treated with 1,5mg of plasmid DNA (four electroporation treatments in each lumbar muscle, 100 µl per site) or with 400 µg of recombinant FcBoNT/A homogenized in 0.5 ml of Freund’s complete adjuvant (first treatment) or Freud's incomplete adjuvant (2nd and 3rd treatments) delivered intramuscularly in the lumbar muscle. Total antibody titers were determined by ELISA of serum samples at different time points of the treatment (days 30, 45, 60, 75, and 90) and represented as gray histograms for the DNA electroporation treatment and black histograms for the recombinant protein immunization. Results show mean ± SEM values. * (p < 0.05) indicates a significant difference between total antibody titers obtained with protein injections and those obtained with plasmid electroporation. The neutralizing titers at the different time points are indicated in bold characters above the corresponding histogram.
Further improvements in our electroporation protocol, obtained by modulating the delivered plasmid dose and the electrodes allowed us to set up our best conditions: three electroporation treatments of rabbits at days 0, 30 and 60 with a total of 3.5 mg of plasmid DNA per treatment, and using 3-needles electrodes (gift of Sphergen). DNA delivery was performed by injecting 100 µl of the plasmid solution in 4 different sites of each lumbar muscle, plus two sites in each thigh. Using this protocol, the highest neutralizing titer against botulinum toxin A obtained with the BTX electrodes was 50 IU/ml, while it reached 200 IU/ml with the Sphergen electrodes, leading to use these latter device in further experiments (data not shown). To assess whether it was possible to raise neutralizing antibodies against other botulinum toxin serotypes in rabbits or not, we applied these best conditions to rabbits treated with plasmids encoding FcBoNT/B and FcBoNT/E, respectively. Three rabbits were treated for each antigen, and total antibody titers and neutralizing titers were followed over time. Results are shown in Figure 3. With pVax-FcBoNT/B plasmid, the total antibody titers remained at low levels (below 1000) even at day 75 which usually leads to the highest titer value, and neutralizing titers reached a maximum of 1 IU/ml at day 75 (Fig. 3A) compared with a titer of 200 IU/ml obtained with pVax-FcBoNT/A. With pVax-FcBoNT/E, total antibody titer was about 2000 at days 45 and 75, and the neutralizing titers ranged from 2 IU/ml to 20 IU/ml according to the rabbit (Fig. 3B).
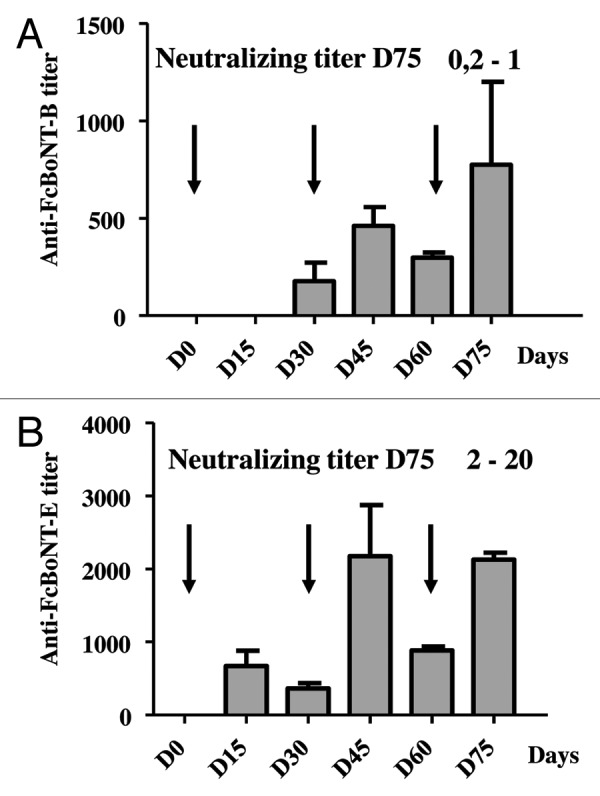
Figure 3. Antibody responses of rabbits treated with plasmid pVax-FcBoNT/B or pVax-FcBoNT/E followed by electroporation at days 0, 30 and 60 as indicated by the arrows using the Sphergen power supply device. New Zealand white rabbits from Hypharm/Groupe Grimaud (n = 3) were treated three times with 3.5 mg of plasmid pVax-FcBoNT/B (A) or with 3.5 mg of plasmid pVax-FcBoNT/E (B). Total antibody titers were determined by ELISA of serum samples at days 15, 30, 45 60, and 75 of the treatment and represented as histograms. Results show mean ± SEM values. The neutralizing titers at day 75 are indicated in bold characters.
High-titer neutralizing antiserum in rabbits
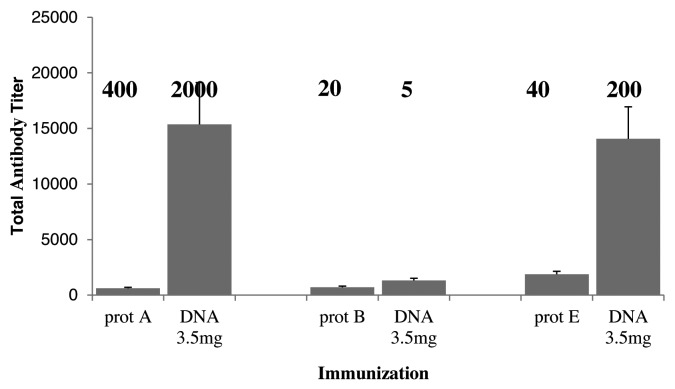
The European Pharmacopoeia corresponds to 500 IU/ml for botulinum toxins A and E, and 50 IU/m for botulinum toxin B. In order to investigate whether our protocol could lead to neutralizing antiserum fitting the requirements of the European Pharmacopoeia, New-Zealand white rabbits selected for a high antibody response were used (HY07 rabbits from Hypharm/groupe Grimaud). Four rabbits per group were treated by DNA electroporation of 3.5 mg of antigen encoding plasmid, or with intramuscular injection of 400 µg of the corresponding recombinant protein for each botulinum toxin type A, B or E. Four rabbits were also electroporated with a mix of the three antigens. Treatments were performed at days 0, 30 and 60. Blood samples were harvested at day 75 for total antibody titer and neutralizing titer determination. The results are shown in Figure 4. For FcBoNT/A antigen, the recombinant protein treatment led to a neutralizing titer of 400 IU/ml, although the total antibody titer was quite low (about 1000). In contrast, the DNA electroporation treatment led to a very high total antibody titer at day 75 (15 000), and to a neutralizing titer of 2000 IU/ml. Similarly, treatment with the recombinant FcBoNT/E antigen led to a low total antibody titer (2000) and a neutralizing titer of 40 IU/ml while the DNA electroporation treatment led to a total antibody titer of 14000 and a neutralizing titer of 200 IU/ml. However, in the case of the FcBoNT/B antigen, all total antibody titers were low (under 1000), and the neutralizing titer was higher when the recombinant protein was used (20 IU/ml) than with the DNA electroporation treatment (only 5 IU/ml).
Figure 4. Antibody responses of Hypharm/Groupe Grimaud rabbits immunized with plasmid pVax-FcBoNT/A with electroporation or recombinant protein A, pVax-FcBoNT/B with electroporation or recombinant protein B, pVax-FcBoNT/E with electroporation or recombinant protein E, at days 0, 30, and 60. New Zealand white rabbits (Hypharm/Groupe Grimaud, France, n = 4) were treated with 3.5mg of plasmid pVax-FcBoNT with electroporation, or intramuscularly with 400µg of the corresponding recombinant FcBoNT homogenized in 0,5ml of Freund’s complete adjuvant (first treatment) or Freud's incomplete adjuvant (2nd and 3rd treatments). Antibody titers were determined by ELISA of serum samples at day 75 after treatment and represented as histograms. Prot A, prot B and prot E means immunization with the recombinant protein respectively corresponding to FcBoNT/A FcBoNT/B and FcBoNT/E antigens. DNA means a DNA electroporation treatment with the corresponding plasmid. Results show mean ± SEM values. The neutralizing titers were also determined at day 75 and are indicated in bold above the corresponding histogram..
When rabbits were immunized by DNA electroporation of a mix of the three antigen encoding plasmids in the hope of raising a trivalent neutralizing antiserum, all neutralizing titers dropped as compared with the single antigen treated rabbits: 200 IU/ml, 2 IU/ml and 5 IU/ml, respectively, for botulinum toxins A, B and E data not shown).
Immunodetection of FcBoNT/A in transfected cells and electroporated mouse muscle
To investigate whether the antibodies raised in rabbits would be valuable tools for immunohistochemistry analysis, B16 mouse melanoma cells were transfected with pVax-FcBoNT/A. B16 melanoma cells (mouse melanoma, ATCC RL-6323) is routinely used in our laboratory to check the expression of plasmid DNA vectors, as these cells are easy to transfect. Twenty-four hours after transfection, cells were fixed, and the immunized rabbit serums were used as primary antibodies for detection of the FcBoNT/A protein expression. Control experiment with the day 0 serum that does not contain any anti-toxin antibody showed no background labeling (Fig. 5A, panel 2) while using the day 75 serum allowed strong labeling of the transfected cells (Fig. 5A, panel 4). To assess the efficacy of the rabbit polyclonal serum in vivo, mice were electroporated with pVax-FcBoNT/A, and sacrificed at day 3. Muscles were harvested and sliced, and the rabbit serums were used for detection of the FcBoNT/A expressing myofibers (Fig. 5B). While the control experiment with non-electroporated muscle (panel 1) or electroporated muscle with a day 0 serum (not shown) showed no labeling, FcBoNT/A was nicely detected with the day 75 serum (panel 2). Therefore, not only the rabbit serums are highly neutralizing against botulinum toxin A, but they are also suitable for in vitro and in vivo immunohistochemistry.
Figure 5. Antibody detection of FcBoNT/A in transfected cells and electroporated mouse muscles. (A) B16 melanoma cells were transfected with pVax-FcBoNT/A and fixed 24h post transfection. Nuclei DAPI staining of the cells is shown in blue (A1 and A3). Immunodetection of FcBoNT/A was performed with naïve rabbit serum (day 0, A2) or immunized rabbit antiserum at day 75 (A4) as primary antibodies. The labeled secondary antibody used for evelation is a goat anti-rabbit IgG conjugated to Alexa Fluor® 488 and appears in green. (B) Immunodetection of FcBoNT/A in a naïve mouse skeletal muscle (1) and a pVax-FcBoNT/A electroporated mouse muscle at day 3 (2) with a day 75 rabbit antiserum used as primary antibody. The labeled secondary antibody used for revelation is a goat anti-rabbit IgG conjugated to Alexa Fluor® 488 and appears in green.
Discussion
Botulinum neurotoxins are considered the most potent toxins in humans. Besides their clinical use for a variety of chronic conditions,21,22 they can be lethal if ingested accidently with contaminated food and are also classified by the Centers for Disease Control and Prevention (CDC) as Category A biothreat agents. As no therapeutic drugs are available, the most suitable current treatment is based on a passive immunotherapy using toxin neutralizing antibodies.23 Although monoclonal neutralizing antibodies might be a viable alternative and have shown some efficacy in mouse protection assays,24-26 the current treatments rely on the use of polyclonal antibodies generally produced by immunization of animals (or humans for BabyBIG) with toxoids, which requires the handling of the botulinum toxins and a whole process of formalin detoxification. However, the 50 kDa non-toxic C-terminal part of the heavy chain has been shown to be very immunogenic and able to elicit a protective immune response in animals immunized with the recombinant protein,27-30 making it an excellent candidate for producing neutralizing antibodies. It still needs the production of the corresponding recombinant protein. In the early 1990s, the administration of a naked DNA plasmid encoding an antigen was shown to elicit both humoral31 and cellular32 immune responses. Since then, DNA immunization has been largely used in a variety of preclinical models, including both infectious (either viral, bacterial or parasitic) or acquired diseases, such as cancer (reviewed in refs. 17, 33–34) or autoimmune diseases35 and allergy.36 Intramuscular DNA electroporation has been shown to greatly enhance the immunogenicity and vaccine efficiency, including in large animals.37,38 It allows a sustained production in muscles for more than several months, so that long-lasting antibody production is expected in the treated animals. DNA immunization has already been used in mice to elicit an immune response against botulinum toxins F, A or C,14,39,40 but with relatively modest neutralizing titers.
DNA immunization is usually used for classical vaccination, aiming at inducing both the humoral and the cellular responses, but rarely focuses on the production of high antibody titer serum. In this article, we have investigated the possibility of high titer neutralizing antiserum production using in vivo intramuscular DNA electroporation in rabbits. We have focused on the production of antisera against BoNT/A, BoNT/B and BoNT/E, which are the most potent forms of BoNT identified so far for humans. We have previously shown that treating mice twice with optimized plasmid DNA encoding the non-toxic C-terminal heavy chain fragment of each toxin fused to a secretion signal would lead to induction of high titer neutralizing antibodies in mice against botulinum toxins A, B and E.18 However, upscale to larger animals is necessary if a large amounts of antibodies is needed. We first optimized our immunization protocol in mice and set up our best conditions to be three plasmid electroporation treatments (8 square wave pulses, 20 ms, 200 V/cm, 5Hz) spaced by 30 d. When used with the pVax-FcBoNT/A plasmid, neutralizing titers against botulinum toxin A as high as 100 IU/ml were obtained at day 75, which is comparable to what was obtained by a classical recombinant protein immunization (Fig. 1). We then decided to upscale the immunization process to rabbits from Hypharm/Groupe Grimaud, France, using commercial 2-needles electrodes from BTX, USA, using a similar treatment. We first demonstrated that a 3-electroporation immunization (8 square wave pulses, 20 ms, 120 V/cm, 5Hz) with 600 µg of plasmid injected in 8 sites of the lumbar muscle elicited a long-term humoral immune response in the rabbits, as antibodies against the antigen could be detected 4 mo after the last treatment (Fig. 2A). Furthermore, the raised antiserum showed a neutralizing activity against botulinum toxin A of 10 IU/ml, which we believed was too low for a high titer antiserum production. Increasing the plasmid dose to 1.5 mg per treatment (still in 8 injection sites) allowed an increase of the total antibody titer and in the neutralizing titer up to 20 IU/ml at days 75 and 90. This was comparable to a classical protein immunization with 400 µg of the recombinant protein administered intramuscularly. A dose study led us to determine that the best DNA plasmid dose was 3.5 mg per treatment (data not shown). In this case, the plasmid was administered in four different sites of each lumbar muscle and in two sites of each thigh, for a total of 12 sites per rabbit. The neutralizing titer then increased to 50 IU/ml. However, we estimated that the BTX electrodes were not very suitable for this rabbit treatment for two reasons: first, they are too thin to pierce the rabbit skin, which makes a skin incision necessary before the treatment. Second, they are only 20 mm long, and of very small diameter, which results in a very local electric field and a reduced transfected area in the muscle. We then moved to three-needle electrodes (gift of Sphergen) made of 50 mm long regular 21G syringe needles. These needles are strong enough to avoid the need of rabbit skin incision, and allow a much larger electric field. We then believed that the muscle transfected area would be larger, resulting in a higher antigen expression and an improved humoral response. When the immunization protocol was repeated with this new device on one rabbit in a preliminary experiment, the total antibody titer was 4000, and the neutralizing antibody titer reached as high as 200 IU/ml against botulinum toxin A, showing that the choice of the electrodes is definitely an important parameter to consider for increasing both the total antibody titer and the neutralizing titer (data not shown). When applied to the Fc fragments of botulinum toxins B and E (n = 3), the same immunization protocol resulted in a lower total antibody titer, and a lower neutralizing titer, compared with botulinum toxin A: no more than 20 IU/ml for botulinum toxin type E, and no more than 1 IU/ml for botulinum toxin B (Fig. 3). A lower neutralization titer has already been reported after 3 immunizations of recombinant Fc fragment type E than with type A,41 and eliciting a highly neutralizing immune response against botulinum toxin B has already proven problematic.42 The promising neutralizing titers obtained against botulinum toxins A and E particularly led us to perform a pilot study at the Industrial Centre de Bioexpérimentation Valbex, France. In this new study, HY07 New-Zealand rabbits that have been selected for the quality of their antibody response were used (personal communication, Hypharm). Four rabbits per group were treated, either with three intramuscular injection of recombinant protein or with 3 electroporation of the antigen encoding plasmid. As compared with the regular New-Zealand rabbits from Hypharm/Groupe Grimaud, we observed a dramatic increase in the total antibody titer (15 000 and 14 000, respectively, for toxins type A and E, vs. 4000 and 2000). Accordingly, the neutralizing titers determined by the mouse lethal assay also dramatically increased and reached 2000 IU/ml against botulinum toxin A and 200 IU/ml against botulinum toxin E (Fig. 4), showing that not only the electrodes, but also the animal strain is a key factor. Comparatively, the neutralizing titers obtained with the corresponding recombinant proteins led to neutralizing titers of 400 IU/ml against botulinum toxin A and 40 IU/ml against botulinum toxin E. Therefore, DNA immunization is a viable alternative for producing high titer antiserum, and the extremely high titers obtained in this experiment indicate that these antibodies have therapeutic potential, and meet the requirement of the European Pharmacopeia.43 The case of botulinum toxin B is quite different: the total antibody titers remained low (1,000), and unlike toxins A and E, the neutralizing titer was higher with the recombinant protein immunization than with the electroporation treatment. Indeed, the neutralizing titer obtained after DNA immunization was only 5 IU/ml while it reached 20 IU/ml with the recombinant protein treatment. For comparison, a titer as high as 220 IU/ml has been reported against toxin B in the rabbit after alkylated toxoid immunization.13 Although it is known that it is difficult to induce a strong immune response against botulinum toxin B, this does not explain the difference we observed between the recombinant FcBoNT/B fragment and the DNA immunization. The reason why the botulinum toxin B Fc fragment has a different behavior than the two other fragments of toxins A and E after genetic immunization remains unclear and needs further investigation. In order to better characterize the antibodies raised in rabbits, an avidity test measuring the overall strength of binding to the antigen was performed in presence of the ammonium thiocyanate chaotropic agent, revealing high avidity antibodies against botulinum toxins A and E, and low avidity antibodies against botulinum toxin B (data not shown), in agreement with the difference observed in the neutralizing titers. Finally, the high avidity of the antibodies against FcBoNT/A led us to use them as primary antibodies for immunodetection of FcBoNT/A in transfected cultured cells and in electroporated muscle. Figure 5 shows that these antibodies are very suitable for both types of detection, with no background.
To conclude, we have demonstrated in the present work that DNA immunization mediated by electroporation performed in rabbits with the right combination of electrodes and animal strain may lead to the induction of a very high titer antibody response. In the case of botulinum toxins A and E, the high neutralizing titers obtained may be compatible with clinical applications. The raised antibodies have also proven useful for immunodetection of protein in transfected cells and in vivo. We then believe that this technique could be used for a wide range of antigen for raising high titer antibodies.
Materials and methods
Plasmids
All plasmids used in this study are based on a pVax1 vector backbone (Invitrogen), in which the original CMV promoter has been replaced with the CMV promoter of the pCMVβ plasmid (Clontech), and have been already described in detail.18 Briefly, all plasmids contain codon optimized sequences based on mouse high frequency codons, and encode the C-terminal fragment of respectively, botulinum toxin A (pVax- FcBoNT/A), botulinum toxin B (pVax-FcBoNT/B) and botulinum toxin E (pVax-FcBoNT/E), fused to the mouse erythropoietin secretion signal.
Plasmids DNA were transformed and produced in E. Coli DH5α strain, and then further purified using Qiagen Megaprep Endo-free kits (Qiagen). All dilutions were done in saline (NaCl 0.9%).
Animal immunization
In vivo mice experiments were performed on 6-week-old SWISS female mice (Janvier, France). Electroporation experiments were performed as previously described.18 Briefly, mice were anaesthetized by intraperitoneal injection of a mix of ketamine (100 mg/kg) and xylazine (10 mg/kg) in 150 mM NaCl. Hind-legs were shaved. 40 µg of plasmid DNA in 30 µl NaCl 0.9% was injected longitudinally, using a syringe into the tibial cranial muscle. After injection, transcutaneous electric pulses were applied by two stainless steel external plate electrodes placed about 5 mm apart, at each side of the leg. Electrical contact with the leg skin was ensured by application of conductive gel. Eight square wave electric pulses of 200 V/cm and 20 ms duration were generated at a frequency of 5 Hz by a Sphergen power supply. For protein immunization, mice were injected intraperitoneally with 1µg of recombinant FcBoNTA protein (produced in BL21 E. coli strain as exposed in ref. 27) homogenized in 1mg aluminum hydroxide (Alugel, Serva) in a 100 µl saline volume. Groups of 5 mice were treated at day 0, 30 and 60. Blood samples were collected by retro-orbital bleeding at the desired time points.
Rabbit experiments were conducted on 14-weeks-old New-Zealand male and female rabbits either from Hypharm/Groupe Grimaud, France or HYPHARM/Groupe GRIMAUD, France. Two types of electrodes were used: 2-needle electrodes from BTX, USA and 3-needle electrodes from Sphergen, France. Rabbits were anaesthetized by intramuscular injection of a ketamine (35 mg/kg) –xylazine (5 mg/kg) mix in 150 mM NaCl, and legs and back were shaved. For BTX electrodes, the skin was incised, and the required amount of DNA plasmid in saline was injected directly in 4 different sites of each longissimus dorsi muscle, and in 2 sites of each thigh if necessary, with a 1 ml syringe (100 µl per site). Electrical pulses (8 pulses, 120 V/cm, 20 ms, 5 Hz) were then delivered at each injection site with the BTX electrodes. These electroporation parameters have been determined in unpublished preliminary experiments to lead to the highest expression in the rabbit muscle. For Sphergen electrodes, the same procedure was performed, except that no skin incision was needed. For protein immunization, 400 µg of the relevant recombinant protein FcBoNT were mixed with 500 µL of Freund’s adjuvant in a total volume of 1ml. 0.5 ml of this mix was injected intramuscularly into the longissimus dorsi muscle. Groups of 4 rabbits were treated at days 0, 30 and 60. Blood samples were collected from the central ear artery at the desired time points.
All experiments were conducted according to the NIH recommendations for animal experimentation. The experiment with the Frères Grimaud Sélection rabbits was performed in the Centre de Bio-experimentation Valbex, France.
Titration of antibodies against FcBoNT in serum
To quantify the antibody responses, serum was collected at various time points by retro-orbital puncture in mice or ear bleed in rabbits and stored at 4 °C before assaying. Microtiter plates were coated overnight at +4 °C with the relevant recombinant FcBoNT as previously described18 and were blocked with PBS-0.1% Tween20–2%BSA (30 min, at room temperature). The plates were washed three times with PBS-0.1% Tween20 and serial 2-fold dilutions of serum samples in PBS-0.1% Tween20–1% BSA (starting at 1:100) were then added (100 µL/well). The plates were incubated for 1 h 30 min at room temperature under agitation and washed three times. Peroxidase-conjugated anti-mouse immunoglobulin (1:2000; 100 µl/well) for mouse sera, or peroxidase-conjugated protein A (1:5000; 100µl/well) for rabbit sera, was added. The plates were incubated at room temperature for 1 h and washed three times with PBS-0.1% Tween20. The revelation was done with orthophenylenediamine (Sigma) and 0.2% H2O2. The reaction was stopped with 3M HCl (50 µL/well) and the absorbance was read at 490 nm with a microplate reader.
Absorbance readings were plotted against the reciprocal of the dilution. The antibody titer of a serum was determined graphically and calculated as the reciprocal of the dilution where the absorbance of the serum was 50% below that of the maximal absorbance.
Mouse protection assays
The neutralizing capacity of mice and rabbit antisera was evaluated in the in vivo mouse lethal toxin challenge assay. Neutralizing tests were performed using botulinum neurotoxin, type A, B or E purchased from Metabiologics, USA. The LD50 of each toxin was experimentally determined. Mice sera were pooled in equal volumes prior to the test. 10-fold serial dilutions of these pooled sera were made in the incubation buffer and were incubated for 30 min at 37°C with the relevant botulinum toxin A, B or E (which was diluted in 50 mM phosphate buffer pH6.5 containing 1% gelatin) at a dose of 10 mouse lethal dose/ml (MLD/ml). One ml of the diluted toxin (corresponding to 10 MLD) was injected intraperitoneally into groups of 5 SWISS male mice (20–25 g). The mice were observed for 4 d and the total deaths and survivals were recorded as already described18. The same test was performed for each rabbit serum.
Detection of FcBoNT-A protein in transfected cells
B16 mouse melanoma cells grown on 15 mm coverslips were transfected with pVax-FcBoNT/A. 24 h after transfection, cells were washed in phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 15 min at room temperature, and permeabilized with 0,05% saponin in PBS containing 0,2% bovine serum albumin overnight at 4°C. IgGs from serums of immunized rabbits were purified with the Melon Gel IgG Purification System (Thermo Scientific) and were used as primary antibodies. Rabbit immunoglobulin G (IgG) were diluted 1:100 in PBS-bovine serum albumin-saponin and incubated for 1 h with fixed cells at room temperature. After washing to remove unbound antibody, cells were incubated for 1 h at room temperature with goat anti-rabbit IgG conjugated to Alexa Fluor® 488 (A-11008, Life Technologies) diluted 1:500. Cells were washed and nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) for 15 min. Finally coverslips were mounted in Mowiol.
Detection of FcBoNT-A protein into the muscle of immunized mice
Tibial cranial muscles of mice were harvested, frozen into a cold isopentan solution and sliced with a cryostat into 10 µm sections. The muscle sections were fixed with 4% para-formaldehyde for 15 min and permeabilized with PBS 1% tritonX100 for 30 min at room temperature. Then, the sections were washed with PBS and incubated with a PBS-5% milk solution for 1 h at room temperature and in a wet chamber. Slices were washed with PBS, and incubated with purified anti-rabbit immunoglobulin (10 times dilution in PBS-1% milk) for 1 h at room temperature in a wet chamber. Next, the slices were washed 3 times for 5 min with PBS, and incubated with a FITC-conjugated rabbit anti-immunoglobulin (1:500 in PBS-1% milk) for 1 h at room temperature in a dark wet chamber. The slides were washed with PBS, stained for 5 min with a DAPI solution, washed again 5 times for 5 min with PBS. The slices were observed with a fluorescence microscope (Axiphot, Zeiss) coupled with a cooled CCD camera (Hamamatsu) and picture software (Samba, Unilog, Meylan).
Statistical analyses
All the results throughout the manuscript are expressed as mean ± SEM (Standard Error of the Mean). Values of the measured parameters were subjected to the variance analysis, and the comparison between treatments was analyzed with an analysis of variance (ANOVA) test, followed by the Fisher’s test. * p < 0.1; ** p < 0.01; *** p < 0.001.
Acknowledgments
This work was partially funded by Sphergen, France, and the French Délégation Générale pour l'Armement (DGA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25192
References
- 1.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, et al. Working Group on Civilian Biodefense Botulinum toxin as a biological weapon: medical and public health management. JAMA. 2001;285:1059–70. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 2.Lindström M, Nevas M, Kurki J, Sauna-aho R, Latvala-Kiesilä A, Pölönen I, et al. Type C botulism due to toxic feed affecting 52,000 farmed foxes and minks in Finland. J Clin Microbiol. 2004;42:4718–25. doi: 10.1128/JCM.42.10.4718-4725.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Middlebrook JL. Production of vaccines against leading biowarfare toxins can utilize DNA scientific technology. Adv Drug Deliv Rev. 2005;57:1415–23. doi: 10.1016/j.addr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Turton K, Chaddock JA, Acharya KR. Botulinum and tetanus neurotoxins: structure, function and therapeutic utility. Trends Biochem Sci. 2002;27:552–8. doi: 10.1016/S0968-0004(02)02177-1. [DOI] [PubMed] [Google Scholar]
- 5.Pellizzari R, Rossetto O, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philos Trans R Soc Lond B Biol Sci. 1999;354:259–68. doi: 10.1098/rstb.1999.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chertow DS, Tan ET, Maslanka SE, Schulte J, Bresnitz EA, Weisman RS, et al. Botulism in 4 adults following cosmetic injections with an unlicensed, highly concentrated botulinum preparation. JAMA. 2006;296:2476–9. doi: 10.1001/jama.296.20.2476. [DOI] [PubMed] [Google Scholar]
- 7.Prigent J, Mazuet C, Boquet D, Lamourette P, Volland H, Popoff MR, et al. Production and characterisation of a neutralising chimeric antibody against botulinum neurotoxin A. PLoS One. 2010;5:e13245. doi: 10.1371/journal.pone.0013245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnon SS, Schechter R, Maslanka SE, Jewell NP, Hatheway CL. Human botulism immune globulin for the treatment of infant botulism. N Engl J Med. 2006;354:462–71. doi: 10.1056/NEJMoa051926. [DOI] [PubMed] [Google Scholar]
- 9.Karalewitz AP, Barbieri JT. Vaccines against botulism. Curr Opin Microbiol. 2012;15:317–24. doi: 10.1016/j.mib.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 10.White DM, Pellett S, Jensen MA, Tepp WH, Johnson EA, Arnason BG. Rapid immune responses to a botulinum neurotoxin Hc subunit vaccine through in vivo targeting to antigen-presenting cells. Infect Immun. 2011;79:3388–96. doi: 10.1128/IAI.00166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pier CL, Tepp WH, Bradshaw M, Johnson EA, Barbieri JT, Baldwin MR. Recombinant holotoxoid vaccine against botulism. Infect Immun. 2008;76:437–42. doi: 10.1128/IAI.00843-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravichandran E, Al-Saleem FH, Ancharski DM, Elias MD, Singh AK, Shamim M, et al. Trivalent vaccine against botulinum toxin serotypes A, B, and E that can be administered by the mucosal route. Infect Immun. 2007;75:3043–54. doi: 10.1128/IAI.01893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Held DM, Shurtleff AC, Fields S, Green C, Fong J, Jones RG, et al. Vaccination of rabbits with an alkylated toxoid rapidly elicits potent neutralizing antibodies against botulinum neurotoxin serotype B. Clin Vaccine Immunol. 2010;17:930–6. doi: 10.1128/CVI.00493-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng M, Xu Q, Elias M, Pichichero ME, Simpson LL, Smith LA. Protective immunity against botulism provided by a single dose vaccination with an adenovirus-vectored vaccine. Vaccine. 2007;25:7540–8. doi: 10.1016/j.vaccine.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rochard A, Scherman D, Bigey P. Genetic immunization with plasmid DNA mediated by electrotransfer. Hum Gene Ther. 2011;22:789–98. doi: 10.1089/hum.2011.092. [DOI] [PubMed] [Google Scholar]
- 16.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23:421–9. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8:108–20. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 18.Trollet C, Pereira Y, Burgain A, Litzler E, Mezrahi M, Seguin J, et al. Generation of high-titer neutralizing antibodies against botulinum toxins A, B, and E by DNA electrotransfer. Infect Immun. 2009;77:2221–9. doi: 10.1128/IAI.01269-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bigey P, Gnidehou S, Doritchamou J, Quiviger M, Viwami F, Couturier A, et al. The NTS-DBL2X region of VAR2CSA induces cross-reactive antibodies that inhibit adhesion of several Plasmodium falciparum isolates to chondroitin sulfate A. J Infect Dis. 2011;204:1125–33. doi: 10.1093/infdis/jir499. [DOI] [PubMed] [Google Scholar]
- 20.Marks WH, Ilsley JN, Dharnidharka VR. Posttransplantation lymphoproliferative disorder in kidney and heart transplant recipients receiving thymoglobulin: a systematic review. Transplant Proc. 2011;43:1395–404. doi: 10.1016/j.transproceed.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 21.Mahajan ST, Brubaker L. Botulinum toxin: from life-threatening disease to novel medical therapy. Am J Obstet Gynecol. 2007;196:7–15. doi: 10.1016/j.ajog.2006.03.108. [DOI] [PubMed] [Google Scholar]
- 22.Bigalke H. Botulinum toxin: application, safety, and limitations. Curr Top Microbiol Immunol. 2013;364:307–17. doi: 10.1007/978-3-642-33570-9_14. [DOI] [PubMed] [Google Scholar]
- 23.Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004;2:695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee J, McCann C, Ofori K, Hill J, Baldwin K, Shoemaker CB, et al. Sheep Monoclonal Antibodies Prevent Systemic Effects of Botulinum Neurotoxin A1 Toxins 2012; 4:1565-81. [DOI] [PMC free article] [PubMed]
- 25.Adekar SP, Takahashi T, Jones RM, Al-Saleem FH, Ancharski DM, Root MJ, et al. Neutralization of botulinum neurotoxin by a human monoclonal antibody specific for the catalytic light chain. PLoS One. 2008;3:e3023. doi: 10.1371/journal.pone.0003023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazuet C, Dano J, Popoff MR, Créminon C, Volland H. Characterization of botulinum neurotoxin type A neutralizing monoclonal antibodies and influence of their half-lives on therapeutic activity. PLoS One. 2010;5:e12416. doi: 10.1371/journal.pone.0012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavallaie M, Chenal A, Gillet D, Pereira Y, Manich M, Gibert M, et al. Interaction between the two subdomains of the C-terminal part of the botulinum neurotoxin A is essential for the generation of protective antibodies. FEBS Lett. 2004;572:299–306. doi: 10.1016/j.febslet.2004.06.094. [DOI] [PubMed] [Google Scholar]
- 28.Middlebrook JL. Production of vaccines against leading biowarfare toxins can utilize DNA scientific technology. Adv Drug Deliv Rev. 2005;57:1415–23. doi: 10.1016/j.addr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Villaflores OB, Hsei CM, Teng CY, Chen YJ, Wey JJ, Tsui PY, et al. Easy expression of the C-terminal heavy chain domain of botulinum neurotoxin serotype A as a vaccine candidate using a bi-cistronic baculovirus system. J Virol Methods. 2013;189:58–64. doi: 10.1016/j.jviromet.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 30.Henkel JS, Tepp WH, Przedpelski A, Fritz RB, Johnson EA, Barbieri JT. Subunit vaccine efficacy against Botulinum neurotoxin subtypes. Vaccine. 2011;29:7688–95. doi: 10.1016/j.vaccine.2011.07.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–4. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 32.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–9. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 33.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ingolotti M, Kawalekar O, Shedlock DJ, Muthumani K, Weiner DB. DNA vaccines for targeting bacterial infections. Expert Rev Vaccines. 2010;9:747–63. doi: 10.1586/erv.10.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue H, Liang F, Liu N, Song X, Yuan F, Luo Y, et al. Potent antirheumatic activity of a new DNA vaccine targeted to B7-2/CD28 costimulatory signaling pathway in autoimmune arthritis. Hum Gene Ther. 2011;22:65–76. doi: 10.1089/hum.2010.110. [DOI] [PubMed] [Google Scholar]
- 36.Chua KY, Kuo IC, Huang CH. DNA vaccines for the prevention and treatment of allergy. Curr Opin Allergy Clin Immunol. 2009;9:50–4. doi: 10.1097/ACI.0b013e3283207ad8. [DOI] [PubMed] [Google Scholar]
- 37.Otten G, Schaefer M, Doe B, Liu H, Srivastava I, zur Megede J, et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine. 2004;22:2489–93. doi: 10.1016/j.vaccine.2003.11.073. [DOI] [PubMed] [Google Scholar]
- 38.Scheerlinck JP, Karlis J, Tjelle TE, Presidente PJ, Mathiesen I, Newton SE. In vivo electroporation improves immune responses to DNA vaccination in sheep. Vaccine. 2004;22:1820–5. doi: 10.1016/j.vaccine.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 39.Bennett AM, Perkins SD, Holley JL. DNA vaccination protects against botulinum neurotoxin type F. Vaccine. 2003;21:3110–7. doi: 10.1016/S0264-410X(03)00260-3. [DOI] [PubMed] [Google Scholar]
- 40.Yu YZ, Zhang SM, Sun ZW, Wang S, Yu WY. Enhanced immune responses using plasmid DNA replicon vaccine encoding the Hc domain of Clostridium botulinum neurotoxin serotype A. Vaccine. 2007;25:8843–50. doi: 10.1016/j.vaccine.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Zichel R, Mimran A, Keren A, Barnea A, Steinberger-Levy I, Marcus D, et al. Efficacy of a potential trivalent vaccine based on Hc fragments of botulinum toxins A, B, and E produced in a cell-free expression system. Clin Vaccine Immunol. 2010;17:784–92. doi: 10.1128/CVI.00496-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torii Y, Tokumaru Y, Kawaguchi S, Izumi N, Maruyama S, Mukamoto M, et al. Production and immunogenic efficacy of botulinum tetravalent (A, B, E, F) toxoid. Vaccine. 2002;20:2556–61. doi: 10.1016/S0264-410X(02)00157-3. [DOI] [PubMed] [Google Scholar]
- 43.European Pharmacopoeia 7.0, 01/2008:0085, 949 http://online.edqm.eu/