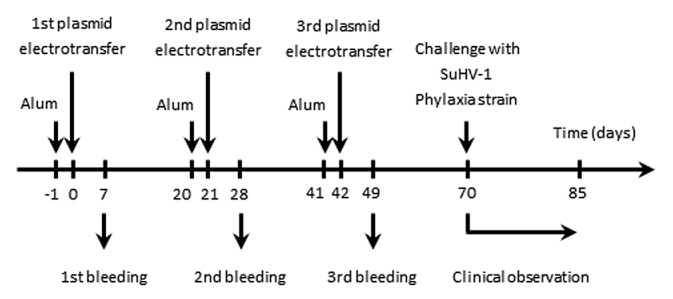
Figure 3. Flowchart of the experiments performed to assess the safety and the efficacy of pStabyCMV-2-gD as a DNA candidate vaccine against Aujeszky’s disease. Mice (n = 10) were immunised by DNA electrotransfer of pStabyCMV-2-gD or pStabyCMV-2 (used as negative control). At the indicated times, blood samples were collected and analyzed for detection of anti-gD antibodies (see Fig. 4A and B). Seventy days after the first plasmid electrotransfer, mice were challenged by injection with the Phylaxia strain of SuHV-1 (see Fig. 4C).
