Abstract
Purpose
Assessment of the integrity of the multifidus muscles and corresponding nerve roots, post-open (OSS) versus minimally invasive spinal surgery (MISS) for lumbar spine fractures.
Methods
We investigated the first six patients undergoing MISS in our institution and age- and sex-matched them with 6 random patients who previously had OSS. All had a similar lumbar fracture configuration without evidence of spinal cord injury. All were assessed using ultrasound muscle quantification and electromyographic studies at a minimum of 6 months post-operatively. Mean cross-sectional area (CSA) was measured at sequential levels within and adjacent to the operative field. Concentric needle electromyography was performed at instrumented and adjacent non-instrumented levels in each patient.
Results
Mean CSA across all lumbar multifidus muscles was 4.29 cm2 in the MISS group, 2.26 cm2 for OSS (p = 0.08). At the instrumented levels, mean CSA was 4.21 cm2 for MISS and 2.03 cm2 for OSS (p = 0.12). At non-instrumented adjacent levels, mean CSA was 4.46 cm2 in the MISS group, 2.87 cm2 for OSS (p = 0.05).
Electromyography at non-instrumented adjacent levels demonstrated nerve function within normal limits in 5/6 levels in the MISS group compared to 1/6 levels in the OSS (p = 0.03). Instrumented levels demonstrated nerve function within normal limits in 5/12 levels in the MISS group compared with 4/12 in the OSS group, including moderate–severe denervation at 5 levels in the OSS group (p = 0.15).
Conclusions
Posterior instrumented MISS demonstrates a significantly superior preservation of the medial branch of the posterior ramus of the spinal nerve and less muscle atrophy, particularly at adjacent levels when compared to OSS.
Keywords: Minimally invasive, Multifidus, Spine, Surgery, Pedicle screw, Electromyography, Fracture
Introduction
Posterior instrumented lumbar fixation of lumbar fractures is performed to correct segmental collapse and permit early ambulation. Open spinal surgery (OSS) or more recently, minimally invasive spinal surgery (MISS) for spinal fractures involves pedicle screw insertion to the levels cephalad and caudal to the fracture to achieve this. The most medial of the paraspinal muscles, the multifidus, has an important role in intervertebral stability. It is innervated by the medial branch nerve (MBN) of the posterior ramus of the spinal nerve at each level, which exits the spinal canal supero-lateral to the facet joint [1]. Unisegmental innervation of multifidus impedes compensation from surrounding paraspinal muscle and damage through direct trauma or traction can cause local denervation [2]. Difficulties post-spinal surgery has been demonstrated with atrophy of this muscle manifesting in kyphotic deformity, poor rehabilitation and chronic pain or “failed back syndrome” [3–5].
A detailed knowledge of the anatomy of the spine is required for any form of pedicle screw fixation [6]. OSS requires stripping of the paraspinal musculature from the midline and direct visualization of the screw insertion points. OSS also permits intervertebral fusion, between the affected levels, which augments the long-term construct. During OSS, the paraspinal muscles, including multifidus undergo intra-operative, often prolonged, retraction and mobilization. This may result in muscle ischemia and posterior ramus degeneration [5, 7, 8]. MISS requires mini-incisions for insertion of blunt dilators onto each screw insertion point. The limited visual field in MISS mandates the visuospatial ability to use radiographic images to guide insertion of the pedicle screw while acknowledging the neural and vascular structures in the region of the pedicles and vertebral body.
The advantages of using MISS over OSS have been demonstrated including less tissue injury [9], reduced length of stay and wound complications [10, 11]. Direct neuromuscular activity comparison between MISS and OSS has not been evaluated post-operatively in lumbar facture patients. We aimed to evaluate integrity of the multifidus muscles and corresponding nerve roots, OSS versus MISS for lumbar spine fractures using ultrasonography muscle quantification and electromyography.
Materials and methods
Patient selection and randomization
MISS was introduced in our institution in Jan 2009 for lumbar fractures and trialed using eight consecutive patients. All patients had fractures confirmed on radiography and CT scanning. All patients had a type A fracture (AO fracture classification) with >30 % anterior height collapse. Two patients who underwent MISS declined to be part of the study, as both cases refused needle electromyography and were excluded leaving six patients for assessment. Six random patients who were recently treated with OSS with similar fracture patterns, and age- and sex-match permitting (Table 1) were included in the study. All 12 patients were evaluated retrospectively and attended for further clinical evaluation, needle electromyography and ultrasonography. No patient had evidence of spinal cord pathology or radicular deficit pre- or post-operatively. Ethics approval was granted by the hospital research board with written consent from each patient. Given the discomfort associated with needle electromyography, three needle sites were permitted per patient.
Table 1.
Study demographics
| Age, gender, initial | AO class | # Level | Method fixation | Levels of fixation | EMG levels | US levels (R & L) |
|---|---|---|---|---|---|---|
| 18, F | A3.2 | L4 | MISS/kyphoplasty | L3–L5 | LL2, LL3, RL5 | L2–S1 |
| 69, F | A3.2 | L1 | MISS/kyphoplasty | T12–L2 | LT12, RL2, LL3 | T11–L4 |
| 55, F | A3.1 | L1 | MISS/kyphoplasty | T12–L2 | LT12, LL2, LL3 | T11–L3 |
| 51, M | A3.1 | L1 | MISS/kyphoplasty | T12–L2 | LT12, RL2, RL3 | T11–L4 |
| 45, M | A3.2 | L4 | MISS/kyphoplasty | L2–S1 | RT12, RL5, RS1 | T12–S1 |
| 43, M | A3.2 | L5 | MISS | L4–S1 | LL2, LL4, RL4 | L2–S1 |
| 44, M | A3.1 | L1 | OSS | T12–L2 | RT12, RL2, RL3 | T11–L3 |
| 60, M | A3.1 | L1 | OSS | T11–L3 | LT12, RL2, RL4 | T11–L4 |
| 48, M | A3.1 | L2 | OSS | T12–L4 | RT12, RL2, RL3 | T11–L4 |
| 62, M | A3.1 | L1 | OSS | T11–L3 | LT12, LL2, RL4 | T11–L4 |
| 49, F | A3.2 | L1 | OSS | T12–L2 | LT11, RT12, LL2 | T11–L3 |
| 60, F | A3.2 | L3 | OSS | L2–L4 | L1–L5 |
Surgical technique
Surgery was performed by three spinal surgeons in the trauma unit. Each procedure was done under general anesthesia with the patient in the prone position. The fractured level was identified fluoroscopically. OSS was performed through a midline approach with exposure of the facet joints of the levels requiring pedicle screw fixation and laterally to the transverse processes to allow application of autogenous bone graft. OSS patients without bone grafting and fusion were not recruited for this study. Using these anatomical landmarks, pedicle screws were inserted under direct vision. Rods were applied with the addition of a cross-connector (Medtronic, CD Horizon® Legacy™, Memphis, TN, USA). MISS was performed through a midline skin incision with mobilization of the skin to either side to facilitate entry of a trocar and series of blunt muscle dilators through a Wiltse approach (Medtronic, CD Horizon® Longitude® Memphis, TN, USA). Pedicle cannulation was guided with fluoroscopy. The rod was then introduced through a caudal or cephalad percutaneous incision.
During the post-operative period, patients were braced as a precautionary measure, maintained on 24 h antibiotics and started on a graduated physiotherapy regime. All patients received post-operative physiotherapy for 5 days as an inpatient and regularly for 6 months as an outpatient.
Cross-sectional area
Cross-sectional area (CSA) was measured by the primary author (DC), proficient in ultrasonography and blinded at the time of assessment to the details of the surgery. Each subject was placed lying relaxed in a prone position and an inclinometer was used to ensure the lumbar spine was within 10° of the horizontal plane, as described by Hides et al. [12]. Sonography was done with a handheld ultrasound system in real time with a 5-MHz curved-array transducer, a 5-cm footprint and 7-cm wave depth. This was performed at all instrumented levels and one level cranial and caudal to the area of instrumentation. Images of the multifidus were obtained from para sagittal orientations to define each level and the corresponding levels were marked. Transverse orientations were obtained at each level to measure the CSA (Fig. 1). Anteroposterior and mediolateral dimensions were used purely as a control measure to ensure low intra-observer error. The transducer was also angled towards each side to enhance accurate measurement of the lateral borders. CSAs for left and right sides were then added and each level was represented as a total. The levels indicated for EMG evaluation were identified and the overlying skin was marked.
Fig. 1.
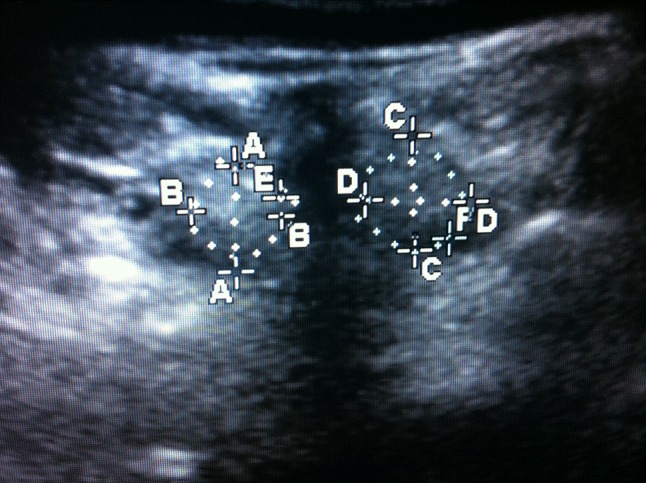
Ultrasound image in the transverse plane with multifidus muscle encircled either side of the posterior spinous process. Anteroposterior (AA, CC) and mediolateral (BB, DD) dimensions and cross-sectional area (CSA: E, F) from both left and right sides were added together to represent CSA at each level
Electromyography
Electromyography (EMG) was performed by an experienced neurophysiologist, with a special interest in neuromuscular injury unconnected with the surgery and blinded to the type of surgery. Concentric needle EMG analysis of the paraspinal muscles was performed using the technique of Haig et al. [13], to facilitate localization of the multifidus muscles at each level tested. EMG was performed at an adjacent non-instrumented site and at two instrumented sites, where pedicle screws were inserted, both one level cephalad and caudal to the fracture. The EMG activity was measured in four quadrants using a 26 gauge concentric EMG needle (38 × 0.45 mm Neuroline Concentric, Ambu, Denmark). A Keypoint EMG System (Dantec Medical, Denmark) was utilized with standard filter settings (0.5–10 kHz), sweep speed of 10 ms/division and gain of 50 μV to 1 mV. EMG activity was evaluated initially at rest and during various stages of activation where fibrillations (Fib), positive sharp waves (PSW), amplitude (Amp), duration, polyphrasia (PP), interference pattern (IP), recruitment (Recruit) and firing was recorded. Patterns of spontaneous EMG activity were documented and graded. The impression of electromyographic findings was based on the summation of the waveforms and the patterns of activation and recruitment (Table 2).
Table 2.
Needle electromyography at selected spinal levels
| Patient | TYPE | SITE | FIB | PSW | Amp | Dur | PP | IP | Recruit | Firing | Denervation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MISS | ADJ | 0 | 0 | + | + | + | – | N | N | Mild, chronic |
| 1 | MISS | INS 1 | 0 | 0 | N | N | + | N | N | N | None |
| 1 | MISS | INS 2 | 0 | 0 | + | + | + | N | LATE | 1+ | Mild, chronic |
| 2 | MISS | ADJ | 0 | 0 | N | N | N | N | N | N | None |
| 2 | MISS | INS 1 | 0 | 0 | N | N | N | N | N | N | None |
| 2 | MISS | INS 2 | 0 | 0 | N | N | N | N | N | N | None |
| 3 | MISS | ADJ | 0 | 0 | N | N | N | N | N | N | None |
| 3 | MISS | INS 1 | 0 | 0 | N | N | N | N | N | N | None |
| 3 | MISS | INS 2 | 0 | 0 | + | + | + | – | LATE | 1+ | Mild, chronic |
| 4 | MISS | ADJ | 0 | 0 | N | N | N | N | N | N | None |
| 4 | MISS | INS 1 | 0 | 0 | + | + | + | – | LATE | 1+ | Mild, chronic |
| 4 | MISS | INS 2 | 0 | 0 | N | N | N | N | N | N | None |
| 5 | MISS | ADJ | 0 | 0 | N | N | N | N | N | N | None |
| 5 | MISS | INS 1 | + | 0 | + | + | ++ | – | LATE | 1+ | Mild, chronic |
| 5 | MISS | INS 2 | ++ | 0 | N | + | N | – | LATE | 1+ | Severe, acute |
| 6 | MISS | ADJ | 0 | 0 | N | N | N | N | N | N | None |
| 6 | MISS | INS 1 | 0 | 0 | + | + | + | – | LATE | 1+ | Mild, chronic |
| 6 | MISS | INS 2 | 0 | 0 | + | + | + | – | LATE | 1+ | Mild, chronic |
| 7 | OSS | ADJ | ++ | + | + | + | + | – | LATE | 1+ | Severe, subacute |
| 7 | OSS | INS 1 | 0 | 0 | N | N | N | N | N | N | None |
| 7 | OSS | INS 2 | + | 0 | +++ | +++ | +++ | – | LATE | 1+ | Severe, chronic |
| 8 | OSS | ADJ | ++ | + | + | + | + | – | LATE | 1+ | Moderate, subacute |
| 8 | OSS | INS 1 | 0 | 0 | N | N | N | N | N | N | None |
| 8 | OSS | INS 2 | ++ | + | + | + | + | – | LATE | 1+ | Severe, chronic |
| 9 | OSS | ADJ | 0 | 0 | + | + | + | – | LATE | N | Mild, chronic |
| 9 | OSS | INS 1 | 0 | 0 | + | ++ | N | – | LATE | 1− | Moderate, chronic |
| 9 | OSS | INS 2 | 0 | 0 | + | ++ | N | – | LATE | 1− | Moderate, chronic |
| 10 | OSS | ADJ | + | 0 | ++ | ++ | + | – | LATE | N | Mild, chronic |
| 10 | OSS | INS 1 | 0 | 0 | N | + | + | N | N | N | Mild, chronic |
| 10 | OSS | INS 2 | +++ | ++ | + | + | + | – | LATE | 1+ | Moderate, subacute |
| 11 | OSS | ADJ | 0 | 0 | N | N | N | N | N | N | None |
| 11 | OSS | INS 1 | + | 0 | + | + | + | – | LATE | 1+ | Mild, chronic |
| 11 | OSS | INS 2 | 0 | 0 | N | N | + | N | N | N | None |
| 12 | OSS | ADJ | 0 | 0 | + | + | + | – | LATE | 1+ | Mild, chronic |
| 12 | OSS | INS 1 | 0 | 0 | + | + | ++ | – | LATE | 1+ | Mild, chronic |
INS 1 instrumented cephalad site, INS 2 instrumented caudal site, ADJ non-instrumented adjacent site, NORM normal, +/−mild abnormality, ++/−− moderate abnormality, +++/−−− severe abnormality, FIB fibrillations, PSW positive sharp waves, Amp amplitude, PP polyphasia, IP interference pattern, Dur duration, Recruit recruitment, firing
Statistics
Statistical analysis was performed using SPSS version 11.5 for Windows XP. Statistical analysis of ultrasound measurements (CSA) at all levels, instrumented levels and non-instrumented adjacent levels incorporated group, gender and spinal level effects based on recent relevant literature [14]. For CSA analysis, box plots were constructed and linear mixed effects models were fitted to obtain an estimate and confidence interval for the effect size on surgery type. A p value of <0.05 (two-tailed test) was considered to be significant. All measurements were done in triplicate and averaged. Cohen’s kappa was used for discrete variables in evaluating intra-observer agreement. A κ value of >0.65 for intra-observer agreement was achieved. EMG analysis: for statistical purposes, the summated level of denervation was evaluated independently, as it reflects the level of axonal injury. Normal was attributed a value of 0, mild as 1, moderate as 2, and severe as 3. Two sample independent t tests were performed for instrumented and non-instrumented adjacent levels.
Results
Demographics
The final cohort included 12 consecutive patients who had ultrasound and electromyography testing (Table 1). Mean age for MISS and OSS was 47 (SD 16.9) and 53.8 (SD 7.7) years, respectively. The MISS group contained three male, three female; the OSS group contained four male, two female patients (Table 1). Assessments were carried out at a minimum of 6 months post-operatively. Mean follow-up was 12 months (SD 5 months) for MISS and 25 months (SD 12 months) for OSS. There were no significant differences in the physical/mental components of the SF36: 43.3/34.7 (SD 3.6/3.2) for MISS and 43.9/31.7 (SD 4.1/5.2) for OSS (p > 0.05). Three-level Cobb angles were measured on pre-operative, intra-operative and post-follow-up radiographs without a significant difference in correction or post-operative kyphosis. None of the patients were treated with or had been planned for removal of their instrumentation during this study.
Ultrasonography
Operated levels were identified by pedicle screw localization, and the multifidus muscle was identified and measured in all patients. The overall mean CSA was 3.22 cm2. The means for MISS and OSS groups were 4.29 and 2.26 cm2 (p = 0.14), respectively. The mean CSAs for instrumented sites were 4.21 cm2 for MISS and 2.03 cm2 for OSS patients (p = 0.24). At non-instrumented adjacent sites, the means for MISS and OSS groups were 4.46 and 2.87 cm2 (p = 0.04), respectively.
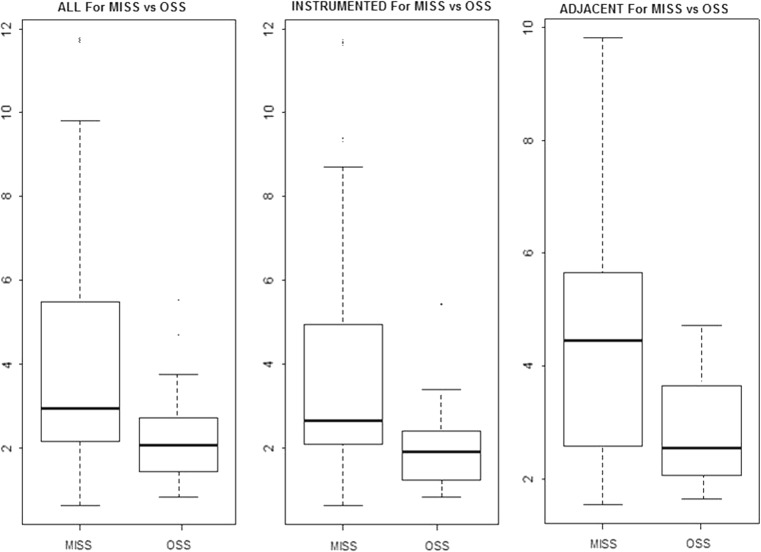
When normalized for age, spinal level and gender effects, the means for MISS and OSS groups were 2.92 and 2.08 cm2 (p = 0.08), respectively. The mean CSAs for instrumented sites were 2.63 cm2 for MISS and 1.84 cm2 for OSS patients (p = 0.13). At non-instrumented adjacent sites, the means for MISS and OSS groups were 4.45 and 2.42 cm2 (p = 0.05), respectively (Fig. 2).
Fig. 2.
Boxplot diagrams of CSA for MISS and OSS patients in all, adjacent non-instrumented and instrumented levels. All three models have group, gender and level effects. The five levels in each box plot represent minimum, first quartile, median, third quartile and maximum values
Electromyography
EMG was performed at marked levels as identified on ultrasonography. Eleven patients tolerated three needle site tests and one patient tolerated one non-instrumented site and only one instrumented site (the aforementioned patient was in the OSS group). All eight electromyographically parameters are noted in Table 2.
All 35 analyzed levels demonstrated more pronounced denervation when comparing MISS to OSS (Severity score 0.5 vs 1.3, p = 0.02). Analysis of two level instrumentations exclusively also demonstrated significantly greater denervation in the OSS group (Severity score 0.3 vs 1.13, p = 0.04).
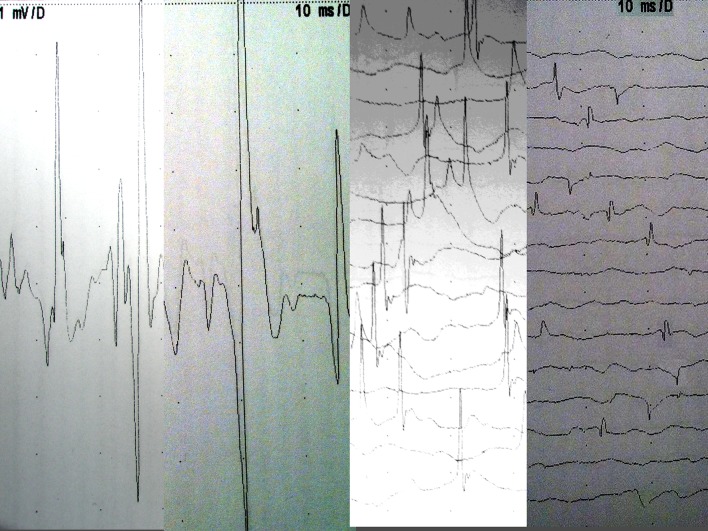
Non-instrumented adjacent levels: a significant difference was noted at non-instrumented adjacent levels between MISS and OSS (Severity score 0.17 vs 1.33, p = 0.03). Denervation was noted in 5/6 OSS levels compared to 1/6 MISS levels. In the OSS group 3/6 had “mild” and 1/6 had “moderate” and 1/6 had severe features of denervation (Table 2). The pattern of abnormalities was consistent with neurogenic change and provided no evidence of myopathy (Fig. 3a–d).
Fig. 3.
a–d Electromyography examples in study patients. a Normal. b Mild chronic change, high amplitude wide complex unit adjacent to normal unit. c Moderate subacute neurogenic change with ongoing prominent fibrillations, moderately reduced interference pattern suggesting ongoing denervation with reinnervation. d Severe neurogenic abnormality with widespread fibrillation potentials
Instrumented levels: significant differences were not as apparent for the instrumented levels (Severity score 0.75 vs 1.36, p = 0.15). In the MISS patients, normal findings were evident at 5/12 levels, mildly abnormal at 6/12 levels, severe changes at 1/12 levels. In the OSS group, normal findings were evident at 3/11 levels, mild abnormalities at 3/11, moderate abnormalities at 3/11 and severe changes at 2/11 levels. Similar to the adjacent groups, abnormal findings were neurogenic in nature.
Discussion
To our knowledge, our current study is the first to confirm clinical neurogenic evidence of MBN injury post-pedicle screw insertion through ultrasonic muscular cross-sectional measurement and concentric needle electromyographical assessment. Pedicle screw fixation through the posterior approach has remained the operative treatment of choice for thoracolumbar and lumbar fractures. OSS has shown risks of poor rehabilitation and low back pain as a result of the iatrogenic injury through this approach [5, 7, 8]. MISS has also demonstrated lower intra-operative time, length of stay, blood loss, wound infection rates, muscle damage and instability of the extensor mechanism [11, 15–17]. Spinal rehabilitation is reliably monitored with the use of ultrasound examination as a measure for muscular cross-sectional area [18–20]. Muscular cross-sectional area accurately represents contractile force potential and strength, which has been reliably mapped on paraspinal electromyography [21, 22]. In this study, eight electromyographical parameters were analyzed on a graded basis so that both the severity and chronicity of denervation and reinnervation could be considered.
Insertion of a pedicle screw frequently traumatizes the neuromuscular unit at that level (61 % of all pedicle screws in this series). Instrumented levels demonstrated normal nerve function in 5/12 levels within the MISS group compared with 3/11 in the OSS group. In the OSS group, moderate to severe denervation was more pronounced, although not statistically significant. (It should be noted however that in one of the MISS patients severe acute/unresolved neurogenic injury was noted at the instrumented level, confirming the known risk following pedicle insertion). Previously, neurogenic injury has been shown in cadaveric specimens where the risk for transection of the MBN has been significantly lower in MISS versus OSS [23]. Prolonged muscular retraction causing ischemia, may also be relevant, but neurogenic findings from our EMG studies indicate that damage to the MBN contributes to a greater proportion of post-operative neuromuscular deficit. Nerve traction, cautery, and dissection with the pedicle cannulation tools are the most likely causes.
Percutaneous pedicle screw insertion has been shown to cause less paraspinal muscle atrophy and weakness than open pedicle screw insertion [24, 25]. Greater muscle damage has also been shown in patients with multilevel exposure [26]. Independent evaluation of adjacent levels has not been reported in the literature. A significant difference was seen at the adjacent non-instrumented levels where MISS demonstrated a greater preservation of innervation and significantly greater muscle bulk. Adjacent segment degeneration in the spine post-fusion is a common problem. Preservation of adjacent innervation may contribute to the preservation of the posterior spinal muscles, which act as a tension band in the maintenance of correct spinal alignment, thus lowering the potential to develop a kyphotic deformity. This may be particularly relevant for spinal injury patients where iatrogenic injury to an otherwise preserved cephalad adjacent level may be less likely with MISS.
A limitation of this study was the small number of patients, especially given the discomfort of the needle electromyography. Pre-operative evaluation was not considered appropriate given the oedema of the paraspinal musculature and potential hyperesthesia at the level of the fracture. Evidence of reinnervation should be present at 6 months post-surgery, indicating a valid time point from when to evaluate patients [27]. Potential for rehabilitation has been shown not to change after this period [28, 29]. Thus the difference in follow-up beyond 6 months did not concern us. The additional bone grafting of OSS, where a wider dissection is required, makes the comparison more complex and the differences cannot be solely due to pedicle screw insertion and retraction. This is especially relevant in light of recent studies that demonstrate that there is no benefit to spinal fusion in fixation of thoracolumbar and lumbar fractures [30, 31]. While none of our patients had their instrumentation removed, some studies suggest routine instrumentation removal in MISS patients, which if required may further precipitate neurogenic injury at the affected levels. Furthermore, the difference in surgical approach may account for some of the differences in outcome. Para-median fascial approach (similar to the MISS approach taken in this study) has been shown to generate less peak pressure and preserve muscle volume when compared to the more traditional midline approach [32, 33]. It is possible that the ability of patients to activate their paraspinal/multifidus muscles following OSS may be hampered by post-operative fibrosis. The pattern of EMG findings however in this group, most likely reflects true neurogenic injury.
Conservatively treated patients were not included in this study. It is possible that EMG abnormalities may be caused by the initial trauma. No focal radicular deficit was noted pre-operatively. Meta-analyses of operative treatment of stable thoracolumbar burst fracture without neurological impairment has not shown major long-term advantage compared with non-operative treatment [34]. However these comparison series are based on traditional OSS as opposed to MISS. Previously, the need for supplemental support with increasing severity of compression, comminution and kyphosis has been reported [35]. Our study did not control for progression of kyphosis, but with better preservation of neurological stability, routine extended instrumentation for more severe fractures may not be as necessary.
In conclusion, we have demonstrated significant neuromuscular differences between MISS and OSS for lumbar fractures. Axonal injury to the medial branch of the posterior ramus at the adjacent segment is significantly reduced with MISS and the multifidus muscle is better preserved. Consideration should be given to these two investigative modalities as further evaluation of paraspinal musculature for post-operative patients undergoing MISS or OSS.
Acknowledgments
Medtronic for funding of co-authors to attend conference. The results of this study were presented at Britspine 2012 and Spineweek 2012.
Conflict of interest
None.
References
- 1.MacDonald DA, Moseley GL, Hodges PW. The lumbar multifidus: does the evidence support clinical beliefs? Man Ther. 2006;11(4):254–263. doi: 10.1016/j.math.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Macintosh J, Valencia F, Bogduk N, et al. The morphology of the human lumbar multifidus. Clin Biomech. 1986;1(4):196–204. doi: 10.1016/0268-0033(86)90146-4. [DOI] [PubMed] [Google Scholar]
- 3.Rantanen J, Hurme M, Falck B, et al. The lumbar multifidus muscle five years after surgery for a lumbar intervertebral disc herniation. Spine. 1993;18:568–574. doi: 10.1097/00007632-199304000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Sihvonen T, Herno A, Paljiarvi L, et al. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18:575–581. doi: 10.1097/00007632-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Gejo R, Matsui H, Kawaguchi Y, et al. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine. 1999;24:1023–1028. doi: 10.1097/00007632-199905150-00017. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein JN, Rydevik BL, Rauschning W. Anatomic and technical considerations of pedicle screw fixation. Clin Orthop Relat Res. 1992;284:34–46. [PubMed] [Google Scholar]
- 7.Styf JR, Willen J. The effects of external compression by three different retractors on pressure in the erector spine muscles during and after posterior lumbar spine surgery in humans. Spine. 1998;23:354–358. doi: 10.1097/00007632-199802010-00014. [DOI] [PubMed] [Google Scholar]
- 8.Nagayama R, Nakamura H, Yamano Y, et al. An experimental study of the effects of nerve root retraction on the posterior ramus. Spine (Phila Pa 1976) 2000;25(4):418–424. doi: 10.1097/00007632-200002150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Kim KT, Lee SH, Suk KS, et al. The quantitative analysis of tissue injury markers after mini-open lumbar fusion. Spine (Phila Pa 1976) 2006;31(6):712–716. doi: 10.1097/01.brs.0000202533.05906.ea. [DOI] [PubMed] [Google Scholar]
- 10.Rahman M, Summers LE, Richter B, et al. Comparison of techniques for decompressive lumbar laminectomy: the minimally invasive versus the “classic” open approach. Minim Invasive Neurosurg. 2008;51(2):100–105. doi: 10.1055/s-2007-1022542. [DOI] [PubMed] [Google Scholar]
- 11.McGirt MJ, Parker SL, Lerner J, et al. Comparative analysis of perioperative surgical site infection after minimally invasive versus open posterior/transforaminal lumbar interbody fusion: analysis of hospital billing and discharge data from 5170 patients. J Neurosurg Spine. 2011;14(6):771–778. doi: 10.3171/2011.1.SPINE10571. [DOI] [PubMed] [Google Scholar]
- 12.Hides JA, Cooper DH, Stokes MJ. Diagnostic ultrasound imaging for measurement of the lumbar multifidus muscle in normal young adults. Physiother Theory Pract. 1992;8(1):19–26. [Google Scholar]
- 13.Haig AJ, Moffroid M, Henry S, et al. A technique for needle localization in paraspinal muscles with cadaveric confirmation. Muscle Nerve. 1991;14(6):521–526. doi: 10.1002/mus.880140606. [DOI] [PubMed] [Google Scholar]
- 14.Hides J, Gilmore C, Stanton W, et al. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Man Ther. 2008;13(1):43–49. doi: 10.1016/j.math.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Payer M. “Minimally invasive” lumbar spine surgery: a critical review. Acta Neurochir (Wien) 2011;153(7):1455–1459. doi: 10.1007/s00701-011-1023-4. [DOI] [PubMed] [Google Scholar]
- 16.Foley KT, Lefkowitz MA. Advances in minimally invasive spine surgery. Clin Neurosurg. 2002;49:499–517. [PubMed] [Google Scholar]
- 17.Mobbs RJ, Sivabalan P, Li J. Technique, challenges and indications for percutaneous pedicle screw fixation. J Clin Neurosci. 2011;18(6):741–749. doi: 10.1016/j.jocn.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Koppenhaver SL, Hebert JJ, Fritz JM, et al. Reliability of rehabilitative ultrasound imaging of the transversus abdominis and lumbar multifidus muscles. Arch Phys Med Rehabil. 2009;90(1):87–94. doi: 10.1016/j.apmr.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Hebert JJ, Koppenhaver SL, Parent EC, et al. A systematic review of the reliability of rehabilitative ultrasound imaging for the quantitative assessment of the abdominal and lumbar trunk muscles. Spine (Phila Pa 1976) 2009;34(23):E848–E856. doi: 10.1097/BRS.0b013e3181ae625c. [DOI] [PubMed] [Google Scholar]
- 20.Wallwork TL, Hides JA, Stanton WR. Intrarater and interrater reliability of assessment of lumbar multifidus muscle thickness using rehabilitative ultrasound imaging. J Orthop Sports Phys Ther. 2007;37(10):608–612. doi: 10.2519/jospt.2007.2418. [DOI] [PubMed] [Google Scholar]
- 21.Haig AJ, LeBreck DB, Powley SG. Paraspinal mapping. Quantified needle electromyography of the paraspinal muscles in persons without low back pain. Spine (Phila Pa 1976) 1995;20(6):715–721. doi: 10.1097/00007632-199503150-00013. [DOI] [PubMed] [Google Scholar]
- 22.Haig AJ, Talley C, Grobler LJ, et al. Paraspinal mapping: quantified needle electromyography in lumbar radiculopathy. Muscle Nerve. 1993;16(5):477–484. doi: 10.1002/mus.880160508. [DOI] [PubMed] [Google Scholar]
- 23.Regev GJ, Lee YP, Taylor WR, et al. Nerve injury to the posterior rami medial branch during the insertion of pedicle screws: comparison of mini-open versus percutaneous pedicle screw insertion techniques. Spine (Phila Pa 1976) 2009;34(11):1239–1242. doi: 10.1097/BRS.0b013e31819e2c5c. [DOI] [PubMed] [Google Scholar]
- 24.Kim DY, Lee SH, Chung SK, et al. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine (Phila Pa 1976) 2005;30(1):123–129. doi: 10.1097/01.brs.0000157172.00635.3a. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Xu HZ, Wang XY, et al. Comparison of the paraspinal muscle change of percutaneous and open pedicle screw fixation in the treatment for thoracolumbar fractures. Zhonghua Wai Ke Za Zhi. 2007;45(14):972–975. [PubMed] [Google Scholar]
- 26.Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine (Phila Pa 1976) 1996;21(8):941–944. doi: 10.1097/00007632-199604150-00007. [DOI] [PubMed] [Google Scholar]
- 27.Datta G, McGregor A, Medhi-Zadeh S, et al. The impact of intermittent retraction on paraspinal muscle function during lumbar surgery. Spine (Phila Pa 1976) 2010;35(20):E1050–E1057. doi: 10.1097/BRS.0b013e3181edea9c. [DOI] [PubMed] [Google Scholar]
- 28.Mannion AF, Denzler R, Dvorak J, et al. A randomised controlled trial of post-operative rehabilitation after surgical decompression of the lumbar spine. Eur Spine J. 2007;16(8):1101–1117. doi: 10.1007/s00586-007-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannion AF, Müntener M, Taimela S, et al. Comparison of three active therapies for chronic low back pain: results of a randomized clinical trial with one-year follow-up. Rheumatology. 2001;40(7):772–778. doi: 10.1093/rheumatology/40.7.772. [DOI] [PubMed] [Google Scholar]
- 30.Sanderson PL, Fraser RD, Hall DJ, et al. Short segment fixation of thoracolumbar burst fractures without fusion. Eur Spine J. 1999;8(6):495–500. doi: 10.1007/s005860050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai LY, Jiang LS, Jiang SD. Posterior short-segment fixation with or without fusion for thoracolumbar burst fractures. a five to seven-year prospective randomized study. J Bone Joint Surg Am. 2009;91(5):1033–1041. doi: 10.2106/JBJS.H.00510. [DOI] [PubMed] [Google Scholar]
- 32.Hyun SJ, Kim YB, Kim YS, et al. Postoperative changes in paraspinal muscle volume: comparison between paramedian interfascial and midline approaches for lumbar fusion. J Korean Med Sci. 2007;22(4):646–651. doi: 10.3346/jkms.2007.22.4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens KJ, Spenciner DB, Griffiths KL, et al. Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. J Spinal Disord Tech. 2006;19(2):77–86. doi: 10.1097/01.bsd.0000193820.42522.d9. [DOI] [PubMed] [Google Scholar]
- 34.Wood K, Buttermann G, Mehbod A, et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003;85(5):773–781. doi: 10.2106/00004623-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 35.McCormack T, KaraiKovic E, Gaines W. The load sharing classification of spine fracture. Spine. 1994;19(15):1741–1744. doi: 10.1097/00007632-199408000-00014. [DOI] [PubMed] [Google Scholar]