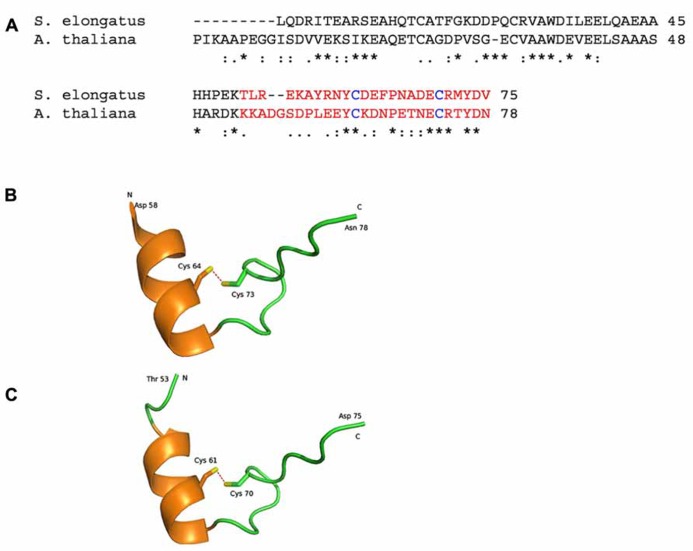
FIGURE 2.
Representations of crystal structures of the Arabidopsis thaliana and Synechococcus elongatus proteins. (A) The amino acid sequence alignment of Q9LZP9 Arabidopsis thaliana CP12-1 (mature protein) and Q31RN5 S. elongatus (strain PCC 7942). The regions in red is represented in the model structures in (B, C). (B) 2.0 Å resolution crystal structure of the C-terminal region of CP12 from Arabidopsis thaliana (Fermani et al., 2012; PDB accession code 3qv1). (C). 2.2 Å resolution crystal structure of the C-terminal region of CP12 from S. elongatus (Matsumura et al., 2011; PDB accession code 3b1j). Each structure contains a single alpha helix and a disulphide bond (red dashed lines). N and C represent the ends of the structured region, with the remaining amino acids being disordered and not modeled. Conserved amino acid residues are denoted by (*); conservative changes (:) and semi-conservative (.).