Abstract
SUMMARY
Patients coinfected with hepatitis C virus (HCV) and the trematode, Schistosoma mansoni, have an increased incidence of viral persistence and accelerated fibrosis. To investigate immunological mechanisms responsible for this more aggressive natural history of HCV, the core HCV-specific T-cell responses were analysed in 44 donated blood units rejected because they had antibodies to HCV (anti-HCV). Half also had anti-S. mansoni antibodies, evidence of past or active infection. HCV-specific ELISPOT responses were examined using pools of 180 overlapping 9-mer peptides with offsets of one covering the core of HCV genotype 4a. Comparison of T-cell responses in blood units positive for both anti-HCV and anti-Schistosoma antibodies with blood units positive only for anti-HCV antibodies showed a significant decrease in core-specific T-cell IFN-γ (505 ± 46 vs. 803 ± 66 ISC/106 cells, P < 0·001), IL-4 (2 ± 108 vs. 641 ± 131 ISC/106 cells, P < 0·001), and IL-10 (159 ± 105 vs. 466 ± 407 ISC/106 cells, P < 0·002) responses. In contrast, there was no significant difference in cell-mediated immune response (CMI) to PHA mitogen between these two groups. Therefore, we concluded T cells from persons with anti-Schistosoma have reduced IFN-γ, IL-4, and IL-10 secreting HCV-specific T-cell responses. This may explain why Schistosoma coinfection increases persistence and severity of HCV infection.
Keywords: cellular immune response, cytokines, hepatitis C, immunomodulation, immunosuppression, schistosomiasis
INTRODUCTION
Schistosomiasis is the second most important parasitic infection with a detrimental socioeconomic impact on more than 200 million people living in developing countries (1). Schistosoma mansoni and Schistosoma haematobium are endemic in many rural areas in Egypt, with community prevalence often ranging between 15–45% (2). Morbidity in humans and experimental animals infected with S. mansoni results primarily from deposition of parasite ova in the hepatic portal tracts with granuloma formation as a result of cell-mediated immunity (CMI) to soluble egg antigen. This may progress to irreversible fibrosis and consequently severe portal hypertension. Severity of disease is partially regulated by the balance of Th1- vs. Th2-type cytokines (3-6), and/or the presence of T regulatory (Tr) cells (7). Well-established S. mansoni infection is characterized by a strong Th2 immunologic bias (3-6), and suppression of the local inflammatory responses in the liver (7-9).
Chronic infection with hepatitis C virus (HCV), the second most important emerging infection and possibly the most important worldwide cause of liver disease, is estimated to be present in 170 million people. Egypt has the highest prevalence of HCV, being 10–25% in most of the same rural areas where schistosomiasis is endemic (10-13).
Natural history of HCV, as well as the mechanisms of viral clearance and persistence, is not entirely understood. HCV causes cirrhosis and/or hepatocellular carcinoma (HCC) in 15–20% of chronically infected individuals (14). The host immune response has a critical role in both control of HCV replication and, just as in the case of schistosomiasis, hepatic injury (15), and evokes CD4+ human leucocyte antigen (HLA) class II-restricted (15,16) and CD8+ HLA class I-restricted CMI (17,18). Analysis of the cytokine profile of bulk cultures, as well as CD4+ T-cell clones, from patients with HCV infection showed viral clearance is associated with a Th1 profile (19,20), and/or IFN-γ producing CD8+ T cell responses (17,21).
Concomitant schistosomiasis and HCV infection is common in Egypt (12,22). Patients with both infections have a higher incidence of cirrhosis and HCC than those matched for age, disease duration, and viral genotype with chronic HCV monoinfection (23). A recent paper reported that Egyptian patients infected with HCV genotype 4 can mount HCV-specific T-cell responses despite the prevalence of concomitant schistosomiasis, but did not offer an explanation for the increased incidence in HCV morbidity observed in the coinfected patients (24).
We hypothesized that immune responses induced by Schistosoma egg deposition in the liver down-regulates the local intrahepatic HCV-specific CMI, consequently promoting persistent viral infection and accelerating the clinical course of HCV, and this might be reflected in the HCV-specific responses in the peripheral blood. To test this hypothesis, we compared peripheral blood mononuclear cells (PBMC) HCV-specific cytokine responses in donated blood units rejected because they were positive for HCV antibodies (anti-HCV) in those with, and without, anti-Schistosoma antibodies.
MATERIALS AND METHODS
Blood units
Forty-four discarded donated blood units from the VASCERA blood bank in Cairo, Egypt, were studied. These blood units were being discarded because they were positive for anti-HCV antibodies using a third generation immuno-assay (EIA). By unlinking the blood units from the donors, the University of Maryland-Baltimore's and Egyptian Ministry of Health & Population's Institutional Review Boards gave us exclusion for studies to standardize our ELISPOT assay. However, this prevented us from utilizing any data about the persons providing the blood or examining their stools or urine for Schistosoma ova. The conduct of this investigation complied with all relevant federal guidelines and institutional policies.
HCV EIA
Quantitative measurement of anti-HCV antibodies response was performed on plasma from the blood units using Ortho HCV 3·0 EIA test system (Ortho Diagnostic System, Raritan, NJ) according to the manufacturer's instructions with some modification and as described (25,26). The level of anti-HCV antibodies was expressed as units in comparison to the positive control serum. One unit of positive control serum is equivalent to the dilution at 50% binding. The results were expressed as units/mL.
HCV RNA polymerase chain reaction
This was quantified by real time polymerase chain reaction (PCR) assay with molecular beacon technology using a Perkin Elmer model 7700, as previously described (27). This methodology was sensitive to approximately 100 RNA molecules/mL, and gives linear results between 102·5 and 107 RNA molecules/mL. Quality control was performed by including 4–10 HCV-negative control sera, and two to four positive sera during each PCR run to monitor the extraction and amplification efficiencies. Assays with positive control quantities outside of the mean ± 2 SD for all assays run (QA curve) were discarded.
Anti-Schistosoma antibodies assay
Dipsticks to detect anti-Schistosoma antibodies were developed utilizing adult worm microsomal antigens GP30 for S. mansoni and GP23 for S. haematobium as previously described (28-31). The sensitivity and the specificity of the assay has been reported 80% and 95%, respectively (32,33). These recombinant proteins were dotted onto nitrocellulose paper (NC; Bio-Rad, Hercules, CA) at a concentration of 0·1 μg/μL phosphate-buffer saline (PBS), using a Bio-Rad sheet Mini-Protean II multi-screen apparatus. Antigen-free PBS and normal human serum diluted 1 : 100 were dotted and used as negative and positive controls. Nitrocellulose (NC) was incubated for 2 h at room temperature, washed, dried, attached to a double-face adhesive tape supported with an inert prespex matrix and cut into 2-mm reagent strips. The sticks were incubated in 1:25 diluted plasma from the blood units for 7 min, washed five times with 0·05% PBS-Tween 20 (Sigma, St. Louis, MO), and then incubated with peroxidase conjugated goat antihuman IgG for 7 min. They were then washed and incubated with diaminobenzidine substrate (Sigma Chemical Co., St. Louis, MO) for 2 min. Washing with distilled water and drying at room temperature stopped the reactions. A positive reaction was a dark purple band.
Synthetic HCV core peptides
A panel of 180 overlapping peptides with a length of 9 amino acids and off set of one, derived from the core region of genotype 4a, according to its sequences (34); accession number CAA 72338, was synthesized (Mimotopes Pty Ltd, Clayton Victoria, Australia). Pools of 10 successive overlapping peptides were used at a concentration of 4 nm for each individual peptide, to study recognition by HCV core-specific cytokine secreting cells.
ELISPOT assays
Peripheral blood mononuclear cells (PBMC) were purified from the discarded blood units by Ficoll-Hypaque, washed three times, and counted for functional analysis using ELISPOT assay. These assays were conducted according to the manufacturer's instructions contained in the γ-IFN ELISPOT Kit, IL-4 ELISPOT Kit, and IL-10 ELISPOT Kit (MABTECH, Catalogue numbers M34201-H, M34101-H and M34301-H, Nacka, Sweden) with modifications. Briefly, 96-well nitrocellulose bottomed millititre plates (Millipore, Bedford, MA) were coated with murine anticytokine Mab at concentration of 15 μg/mL in PBS and incubated at 4°C. After 24 h, the plates were washed and blocked with 10% human AB+ serum for 1 h at 37°C. To estimate the number of HCV-specific cytokine ISC, ex vivo unexpanded PBMC, were added at different concentrations (104–105 cells/well) in 100 μL volume of complete medium (RPMI-1640 containing 10% AB+ serum). The peptide pools, containing 10 peptides of 9-mer, were added at a concentration of 4 nm for individual peptide as described (45,46). After an 18-h incubation at 37°C, the plates were washed five times with PBS containing 0·05% (v/v) Tween 20 using an ELISPOT plate washer (Millipore, Bedford, MA). Biotinylated anticytokines Mab (MABTECH) at a concentration of 1 μg/mL in PBS containing 0·05% (v/v) Tween-20 and 1% (w/v) bovine serum albumin (Sigma, St. Louis, MO) was added and the plates were incubated at room temperature for 2 h at 37°C. Plates were then washed five times, and streptavidin-HRP (1:1000) in blocking buffer was added and incubated at room temperature for 2 h, followed by washing five times with washing buffer and the addition of 3-amino-9-ethyl carbazole (AEC) reagent in substrate buffer (Sigma, St. Louis, MO). After developing the spots for 10–15 min, the plates were washed with distilled water and air-dried. The number of spots was enumerated using a computerized assisted ELISPOT image analyser (Zeiss system, Axioplan 2 imaging), and ks elispot 4·2 program (Zeiss), designed to detect spots using predetermined criteria based on size, shape and colourimetric density.
Statistical Analysis
Data were analysed using the equation: Ag-specific ISC/106 cells = no. of ISC in response to Ag/106 cells – no. of ISC in presence of media alone or control peptide/106 cells. HCV-specific ISC was plotted. The cut-off level was calculated as the average number of ISC in the presence of control peptides +2 SD. To eliminate samples with poor viability, those having PHA responses < 2500 ISC/106 cells, equivalent to the mean −2 SD of all the samples tested, were excluded from analysis. For IL-10 ELISPOT analysis, adherent cells were removed from the PBMC before the assay to decrease the background resulting from spontaneous secretion of IL-10 by adherent cells.
Results were expressed as average number of HCV core-specific T cells/106 and were shown as box and whiskers graphs using prism graph pad (GraphPad Software, San Diego, CA), and were analysed by paired Student's t-test. P < 0·05 was considered significant.
RESULTS
Characterization of blood units
The 44 discarded blood units from the VACSERA Blood Bank were positive for anti-HCV antibodies. Thirty-six (81·8%) were also positive for HCV RNA, and 22 (50%) were positive for anti-S. mansoni antibodies using the dipstick assay. There were no differences in the frequency of HCV RNA or its quantitative levels between the blood units with, and without, anti-Schistosoma antibodies (Table 1).
Table 1.
Quantitative levels of anti- hepatitis C virus (HCV) antibodies and HCV RNA in the blood units
| Characterization of blood units | Anti-Schistosoma antibodies (+) | Anti-Schistosoma antibodies (−) |
|---|---|---|
| Anti-HCV antibodiesa,b (Units/mL) | 81·7 ± 119 | 29·4 ± 27 |
| Median | 20·9 | 20·1 |
| HCV-RNAa,b (log10 copies/mL) | 4·9 ± 1·2 | 4·3 ± 1·1 |
| Median | 5·1 | 4·7 |
| Positive units (%) | 17 (77) | 19 (86) |
Values are mean ± standard deviations (22 blood units per group).
No significant differences.
CMI responses to HCV core peptides
To overcome obstacles due to different HLA types of the blood unit donors, we stimulated the T cells isolated from these blood units with 9-mer overlapping HCV core peptides covering the whole core protein of genotype 4a (8). This approach allowed evaluation of CMI to HCV core antigens with different HLA-restrictions.
To evaluate the effect of Schistosoma coinfection (active or inactive infection as antibodies cannot separate present from past infections) on type 1 CMI against HCV core-specific-IFN-γ secretion, ELISPOT assays were performed for each 9-mer core peptide. They were used in overlapping pools of 10 because of the limited number of PBMC available for the study. To validate our approach, comparison studies were conducted with PBMC derived from blood units from volunteers with, and without, anti-HCV antibodies. A significant difference (P < 0·001) between the average ISC responses in PBMC from blood units from persons having, and not having, HCV antibodies were observed (average 803 ± 66 and 236 ± 25 ISC/106, respectively).
After validating our method, we compared the core HCV-specific IFN-γ responses in PBMC from anti-Schistosoma antibodies positive with anti-Schistosoma antibodies negative blood units. PBMC from units positive for anti-Schistosoma antibodies had significantly lower numbers of overall IFN-γ ISC to HCV core peptides in comparison to the anti-Schistosoma antibodies negative blood units (505 ± 46 vs. 803 ± 66 ISC/106 cells, P < 0·001) (Figure 1). However, no significant differences in the responses to PHA between the two groups were observed (data not shown).
Figure 1.
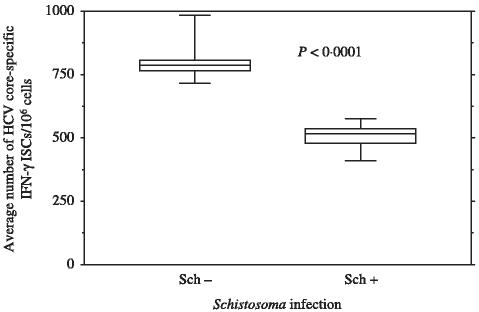
Direct ex vivo IFN-γ ELISPOT for anti-Schistosoma antibodies (+) and anti-Schistosoma antibodies (−) blood units: IFN-γ ELISPOT assay was done as described in the materials and methods section. Eighteen pools were tested corresponding to 180 overlapping core peptides. Plasma from the blood units was tested for viral load and anti-Schistosoma antibodies. Data are presented as the average number of hepatitis C virus (HCV) core-specific direct ex vivo ISC/106 cells in anti-Schistosoma antibodies positive (48) or anti-Schistosoma antibodies negative (57) blood units after subtracting background. The interferon secreting cells (ISCs) values are based on the combined data from all 18 pools of peptides. The box shows the median value and extends from the 25th to the 75th percentile. Whiskers extend down to the smallest value and up to the largest value.
To further characterize the effect of active or inactive Schistosoma coinfection on type 2 immune responses induced by HCV, the core-HCV-specific IL-4, and IL-10-secreting T cells were measured using overlapping 9-mer core peptides. Our data indicated that anti-Schistosoma antibodies positive blood units had significantly lower numbers of core-specific IL-4 (2 ± 108 vs. 641 ± 131 ISC/106 cells, P < 0·001) (Figure 2), and IL-10 (159 ± 105 vs. 466 ± 407 ISC/106 cells, P < 0·002) (Figure 3) in comparison to anti-Schistosoma antibodies negative blood units.
Figure 2.
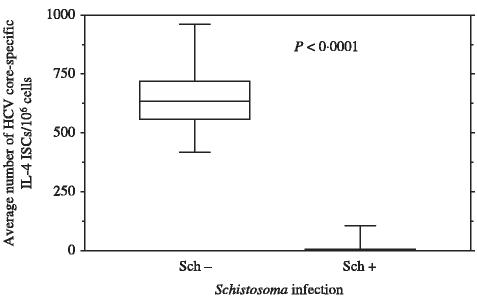
Direct ex vivo IL-4 ELISPOT for anti-Schistosoma antibodies (+) and anti-Schistosoma antibodies (−) blood units: IL-4 ELISPOT assay was done as described in methods. Eighteen pools were tested corresponding to 180 overlapping core peptides. Data are presented as the average numbers of hepatitis C virus (HCV)-core specific direct ex vivo ISC/106 cells in anti-Schistosoma antibodies positive (33) or anti-Schistosoma antibodies negative blood units (44) after subtracting background. The interferon secreting cells (ISCs) values are based on the combined data from all 18 pools of peptides. See legend for Figure 1 for how data is shown.
Figure 3.
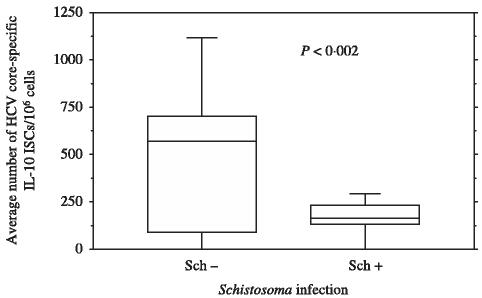
Direct ex vivo IL-10 ELISPOT for anti-Schistosoma antibodies (+) and anti-Schistosoma antibodies (−) blood units: IL-10 ELISPOT assay was done as described in methods. Eighteen pools were tested corresponding to 180 overlapping core peptides. Data are were presented as the average numbers of hepatitis C virus (HCV)-core specific direct ex vivo ISC/106 cells in anti-Schistosoma antibodies positive (44) or anti-Schistosoma antibodies negative blood units (15) after subtracting background. The ISCs values are based on the combined data from all 18 pools of peptides. See legend for Figure 1 for how data is shown.
Analysis of the relationship among the dominant peptide pools and the cytokines secreted by Ag-specific T cells revealed that some peptides pools, e.g. P3 (IFN-γ responses) and P7, P12, P15, P16 for IL-10 responses had significant differences (P < 0·05) between the anti-Schistsoma positive and negative blood units.
DISCUSSION
In the experimental mouse model, chronic infection with S. mansoni causes a shift in T-cell immune responses by increasing the Th2 cytokine, IL-4, while down-regulating the Th1 cytokine, IFN-γ (35,36). Furthermore, dendritic cells (DC) stimulated with Schistosoma-specific phosphatidyserine promoted differentiation of IL-10-producing T regulatory (Tr) cells (7). This finding in animal models was believed by some to be an explanation for the immunosuppression that was sometimes observed in human coinfection with schistosomiasis (4,37). Because CMI is crucial in clearing viral infections, it was natural to investigate concomitant infections with schistosomiasis and hepatotrophic viruses (38). Because the liver is the main target for both pathogens, coinfection with S. mansoni could lead to localized suppression of CMI in the liver and favour viral persistence and more severe hepatic lesions.
Our data indicates a decrease in the IFN-γ secreted by core-specific T cells from blood units having both Schistosoma and HCV antibodies. Others have reported similar dysfunctional HCV-specific T cells that were unable to secret IFN-γ in chronically HCV-infected patients (17,39). In the present study dysfunctional T cells occurred with much greater frequency in PBMC from Schistosoma-coinfected donors than from PBMC from HCV mono-infected donors. A recent report by Kamal et al. of reduced IFN-γ secretion by CD4+ T cells in HCV-Schistosoma coinfected Egyptian patients proposed this as a mechanism by which coinfections with schistosomiasis increases incidence of HCV chronicity and morbidity (40,41). They also noted that coinfections with Schistosoma increased IL-4 and IL-10 (Th2) HCV-specific CD4+ responses (40,41), which differs from the reduced IL-4 and IL-10 T-cell responses observed in our study. They measured T cells responses in PBMC taken from symptomatic HCV-infected patient and not from supposedly asymptomatic blood donors. The role of IL-10-secreting T cells in regulation of immune responses remains unclear (42); however, there is evidence these cells may have regulatory or suppressive functions (43).
Unlinked discarded blood units were used in this study instead of blood samples from HCV-infected patients for several reasons. By unlinking the patient's data from the blood units that would otherwise be discarded, standardization of the ELISPOT could be accelerated since the study was excluded for complete review by the Institutional Review Boards in Egypt and Maryland. Discarded blood units also provided excellent sources of the large numbers of PBMC needed to standardize the ELISPOT, as measuring cytokine profiles of core specific immune responses using 180, 9-mer overlapping core peptides requires at least 2 × 108 cells for each cytokine. Moreover, most previous studies on Schistosoma-HCV coinfected patients were conducted in symptomatic cases, which differ from supposedly asymptomatic blood donors used in the current study. The demographics of schistosomiasis in Egypt makes it probable that the majority of the donors had S. mansoni (2), the species which causes granulomas in the liver and lesions in the intestines and not S. haematobium, the species causing lesions primarily in the bladder and urinary track. With the widespread use of praziquantel therapy to treat schistosomiasis during the past 20 years, it is also likely the majority of the blood donors had inactive or very light infections (2), otherwise they would not be blood donors.
These data using PBMC from anonymous anti-HCV antibodies positive blood units without parasitological documentation of active Schistosoma infection provided opportunity to test our hypothesis that coinfection with Schistosoma down-regulates CMI to HCV. One problem of studying the HLA-restricted immune responses is the heterogenicity of HLA. In addition to different dominant epitopes in each individual, the high mutation rate of HCV complicates the study of HLA-restricted responses. Our approach using 9-mer overlapping peptides with offset of one aa covering the whole core HCV of genotype 4a, the dominant genotype in Egypt, allowed us to measure core-specific responses regardless of the HLA type of the host. However, depletion experiments were not performed to confirm CD8+ T cells were the source of the cytokine secretions. Also, our results suggest only a few peptides pools are associated with certain cytokine profiles secreted by core-specific T cells. This is not surprising given the heterologus nature of the HLA among our Egyptian blood donors. It is remarkable that most of the 18 pools of peptides spanning the whole core protein of HCV stimulated higher responses in the anti-Schistosoma antibodies negative blood. However, because each pool contains 10 peptides, the potential for the presence of dominant peptides in the context of 8 HLA haplotypes of the host within a pool is high.
The core peptides were chosen for three main reasons: (i) the core protein is the most conserved protein in HCV (44,45); therefore, variations in epitopes during HCV mutation are minimized; (ii) the core protein is small (only 180 aa) in comparison to other nonstructural HCV proteins, which makes synthesis of 180 (9-mer) peptides with offset of one aa feasible; and (iii) the core protein is believed to have an important role in the regulation of the immune responses to HCV (46-49). Consequently, studying the immune response to the core protein could define the mechanism of immune regulation in HCV and the effect of coinfections with Schistosoma.
Memory T cells are important in protection from HCV (50-52). We noted a significant decrease in the recall responses of core-specific CD8+ T cells in Schistosoma coinfected patients. This inhibition of the CMI to HCV could be explained by the following mechanisms: (i) a decrease in memory HCV-specific T cells in anti-Schistosoma antibodies positive, in comparison to negative, blood units as suggested by a recent study (53); (ii) induction of T lymphocyte apoptosis and cell death by soluble egg antigen during Schistosoma infection as noted in the experiments using the murine model of schistosomiasis (9,54); and (iii) induction of Tr cells that inhibit local intrahepatic CMI to HCV in Schistosoma infection (7,55).
Despite suppression of CMI in those with anti-Schistosoma antibodies, there were no significant differences in the levels of viremia or anti-HCV antibodies, as previously reported (23,56).
Because the liver is the principal site for both HCV viral replication and egg deposition in Schistosoma infection, we speculated that Schistosoma egg deposition down-regulated the immune responses locally in the liver (8,9,57). This could result in suppression of the intrahepatic bystander immune response to HCV. It is clear this down regulation would be localized since no generalized immunosuppression is reported in Schistosoma-infected patients. Furthermore, no difference in response to PHA in the mono-infected and Schistosoma-coinfected HCV blood units was observed, which further excludes generalized immunosuppression in Schistosoma coinfected HCV patients, and supports the absence of a quality difference in the PBMC between the two groups. These preliminary results have encouraged us to investigate CD4+ and CD8+ immune responses in HCV-infected patients in whom active S. mansoni infections have been parasitogically diagnosed. It is feasible that inactive infections, (i.e. absence of viable egg-laying trematodes in the portal system), could still be producing these immunological changes since the ova remain in the hepatic portal tracks and their soluble antigens could influence CMI for a considerable, but unknown, time.
Acknowledgments
The research was supported in part by NIH grant RO1-AI47349 and the Wellcome Trust-Burroughs Wellcome Fund grants 059113/z/99/a and 059113/z/99/z. We appreciate the assistance of our administrative and technical staffs in Cairo, Baltimore, and New York, particularly Mar Jan Ostrowski and Kelly Weed. Without their help we could not successfully conduct our research.
Glossary
Abbreviations:
- HCV
hepatitis C virus
- HCV-RNA
hepatitis C virus ribonucleic acid
- EIA
enzyme-linked immunoassay
- IL-4
interleukin-4
- PBMC
peripheral blood mononuclear cells
- RT-PCR
reverse transcription polymerase chain reaction
- HLA
human leucocyte antigen
- aa
amino acid
- PBS
phosphate-buffered saline
- NC
nitro-cellulose
- anti-HCV
hepatitis C virus antibody
- IFN-γ
interferon-gamma
- IL-10
interleukin-10
- Mab
monoclonal antibody
- ISC
interferon-γ, IL-4, or IL-10 secreting cells
- PHA
phytohemagglutinin
REFERENCES
- 1.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Khoby T, Galal N, Fenwick A, et al. The epidemiology of schistosomiasis in Egypt: summary findings in nine governorates. Am J Trop Med Hyg. 2000;62:88–99. doi: 10.4269/ajtmh.2000.62.88. [DOI] [PubMed] [Google Scholar]
- 3.Sabin EA, Pearce EJ. Early IL-4 production by non-CD4+ cells at the site of antigen deposition predicts the development of a T helper 2 cell response to Schistosoma mansoni eggs. J Immunol. 1995;155:4844–4853. [PubMed] [Google Scholar]
- 4.King CL, Medhat A, Malhotra I, et al. Cytokine control of parasite-specific anergy in human urinary schistosomiasis. IL-10 modulates lymphocyte reactivity. J Immunol. 1996;156:4715–4721. [PubMed] [Google Scholar]
- 5.King CL, Hakimi J, Shata MT, Medhat A. IL-12 regulation of parasite antigen-driven IgE production in human helminth infections. J Immunol. 1995;155:454–461. [PubMed] [Google Scholar]
- 6.Malaquias LC, Falcao PL, Silveira AM, et al. Cytokine regulation of human immune response to Schistosoma mansoni: analysis of the role of IL-4, IL-5 and IL-10 on peripheral blood mononuclear cell responses. Scand J Immunol. 1997;46:393–398. doi: 10.1046/j.1365-3083.1997.d01-136.x. [DOI] [PubMed] [Google Scholar]
- 7.van der Kleij D, Latz E, Brouwers JF, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 8.Henderson GS, Lu X, McCurley TL, Colley DG. In vivo molecular analysis of lymphokines involved in the murine immune response during Schistosoma mansoni infection. II. Quantification of IL-4 mRNA, IFN-gamma mRNA, and IL-2 mRNA levels in the granulomatous livers, mesenteric lymph nodes, and spleens during the course of modulation. J Immunol. 1992;148:2261–2269. [PubMed] [Google Scholar]
- 9.Lundy SK, Lerman SP, Boros DL. Soluble egg antigen-stimulated T helper lymphocyte apoptosis and evidence for cell death mediated by FasL (+) T and B cells during murine Schistosoma mansoni infection. Infect Immun. 2001;69:271–280. doi: 10.1128/IAI.69.1.271-280.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank C, Mohamed MK, Strickland GT, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Aziz F, Habib M, Mohamed MK, et al. Hepatitis C virus (HCV) infection in a community in the Nile Delta: population description and HCV prevalence. Hepatology. 2000;32:111–115. doi: 10.1053/jhep.2000.8438. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Wahab MF, Zakaria S, Kamel M, et al. High seroprevalence of hepatitis C infection among risk groups in Egypt. Am J Trop Med Hyg. 1994;51:563–567. doi: 10.4269/ajtmh.1994.51.563. [DOI] [PubMed] [Google Scholar]
- 13.Habib M, Mohamed MK, Abdel-Aziz F, et al. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology. 2001;33:248–253. doi: 10.1053/jhep.2001.20797. [DOI] [PubMed] [Google Scholar]
- 14.Seeff LB. Natural history of hepatitis C. Am J Med. 1999;107:10S–15S. doi: 10.1016/s0002-9343(99)00374-5. [DOI] [PubMed] [Google Scholar]
- 15.Cerny A, Chisari FV. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology. 1999;30:595–601. doi: 10.1002/hep.510300312. [DOI] [PubMed] [Google Scholar]
- 16.Diepolder HM, Gruener NH, Gerlach JT, Jung MC, Wierenga EA, Pape GR. Different levels of T-cell receptor triggering induce distinct functions in hepatitis B and hepatitis C virus-specific human CD4 (+) T-cell clones. J Virol. 2001;75:7803–7810. doi: 10.1128/JVI.75.17.7803-7810.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wedemeyer H, He XS, Nascimbeni M, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 18.Koziel MJ, Dudley D, Afdhal N, et al. Hepatitis C virus (HCV)-specific cytotoxic T lymphocytes recognize epitopes in the core and envelope proteins of HCV. J Virol. 1993;67:7522–7532. doi: 10.1128/jvi.67.12.7522-7532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koziel MJ. Cytokines in viral hepatitis. Semin Liver Dis. 1999;19:157–169. doi: 10.1055/s-2007-1007107. [DOI] [PubMed] [Google Scholar]
- 20.Rosen HR, Miner C, Sasaki AW, et al. Frequencies of hepatitis C virus (HCV)-specific effector CD4+ T cells by flow cytometry: correlation with clinical disease stages. Hepatology. 2002;35:190–198. doi: 10.1053/jhep.2002.30293. [DOI] [PubMed] [Google Scholar]
- 21.Thimme R, Bukh J, Spangenberg HC, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci USA. 2002;99:15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strickland GT, Elhefni H, Salman T, et al. Role of hepatitis C infection in chronic liver disease in Egypt. Am J Trop Med Hyg. 2002;67:436–442. doi: 10.4269/ajtmh.2002.67.436. [DOI] [PubMed] [Google Scholar]
- 23.Kamal S, Madwar M, Bianchi L, et al. Clinical, virological and histopathological features: long-term follow-up in patients with chronic hepatitis C co-infected with S. mansoni. Liver Int. 2000;20:281–289. doi: 10.1034/j.1600-0676.2000.020004281.x. [DOI] [PubMed] [Google Scholar]
- 24.Elrefaei M, El-sheikh N, Kamal K, Cao H. Analysis of T cell responses against hepatitis C virus genotype 4 in Egypt. J Hepatol. 2004;40:313–318. doi: 10.1016/j.jhep.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Shata MT, Anthony DD, Carlson NL, et al. Characterization of the immune response against hepatitis C infection in recovered, and chronically infected chimpanzees. J Viral Hepat. 2002;9:400–410. doi: 10.1046/j.1365-2893.2002.00373.x. [DOI] [PubMed] [Google Scholar]
- 26.Shata MT, Tricoche N, Perkus M, et al. Exposure to low infective doses of hepatitis C virus (HCV) induces cellular immune responses without consistently detectable viremia or seroconversion in chimpanzees. Virology. 2003;314:601–616. doi: 10.1016/s0042-6822(03)00461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DH, Prince AM. Automation of nucleic acid extraction for NAT screening of individual blood units. Transfusion. 2001;41:483–487. doi: 10.1046/j.1537-2995.2001.41040483.x. [DOI] [PubMed] [Google Scholar]
- 28.Al-Sherbiny M. Field applicable method for detection of antibodies to Schistosoma species and genus specific antigens using dipsticks. J Egypt Ger Soc Zool. 1996;20:81–97. [Google Scholar]
- 29.Tsang VC, Tsang KR, Hancock K, Kelly MA, Wilson BC, Maddison SE. Schistosoma mansoni adult microsomal antigens, a serologic reagent. I. Systematic fractionation, quantitation, and characterization of antigenic components. J Immunol. 1983;130:1359–1365. [PubMed] [Google Scholar]
- 30.Tsang VC, Hancock K, Kelly MA, Wilson BC, Maddison SE. Schistosoma mansoni adult microsomal antigens, a serologic reagent. II. Specificity of antibody responses to the S. mansoni microsomal antigen (MAMA) J Immunol. 1983;130:1366–1370. [PubMed] [Google Scholar]
- 31.Tsang VC, Hancock K, Maddison SE, Beatty AL, Moss DM. Demonstration of species-specific and cross-reactive components of the adult microsomal antigens from Schistosoma mansoni and Schistosoma japonicum (MAMA and JAMA) J Immunol. 1984;132:2607–2613. [PubMed] [Google Scholar]
- 32.Hancock K, Mohamed YB, Haichou X, Noh J, Dotson EM, Tsang VC. a recombinant protein from Schistosoma mansoni useful for the detection of S. mansoni and Schistosoma haematobium antibodies. J Parasitol. 1997;83:612–618. [PubMed] [Google Scholar]
- 33.Al-Sherbiny MM, Osman AM, Hancock K, Deelder AM, Tsang VC. Application of immunodiagnostic assays: detection of antibodies and circulating antigens in human schistosomiasis and correlation with clinical findings. Am J Trop Med Hyg. 1999;60:960–966. doi: 10.4269/ajtmh.1999.60.960. [DOI] [PubMed] [Google Scholar]
- 34.Chamberlain RW, Adams N, Saeed AA, Simmonds P, Elliott RM. Complete nucleotide sequence of a type 4 hepatitis C virus variant, the predominant genotype in the Middle East. J General Virol. 1997;78((6)):1341–1347. doi: 10.1099/0022-1317-78-6-1341. [DOI] [PubMed] [Google Scholar]
- 35.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Down-regulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheever AW, Jankovic D, Yap GS, Kullberg MC, Sher A, Wynn TA. Role of cytokines in the formation and down-regulation of hepatic circumoval granulomas and hepatic fibrosis in Schistosoma mansoni-infected mice. Mem Inst Oswaldo Cruz. 1998;93((Suppl. 1)):25–32. doi: 10.1590/s0074-02761998000700004. [DOI] [PubMed] [Google Scholar]
- 37.Scott JT, Turner CM, Mutapi F, Woolhouse ME, Ndhlovu PD, Hagan P. Cytokine responses to mitogen and Schistosoma haematobium antigens are different in children with distinct infection histories. Parasite Immunol. 2001;23:519–526. doi: 10.1046/j.1365-3024.2001.00409.x. [DOI] [PubMed] [Google Scholar]
- 38.Marshall MA, Jankovic D, Maher VE, Sher A, Berzofsky JA. Mice infected with Schistosoma mansoni develop a novel non-Tlymphocyte suppressor population which inhibits virus-specific CTL induction via a soluble factor. Microbes Infect. 2001;3:1051–1061. doi: 10.1016/s1286-4579(01)01499-x. [DOI] [PubMed] [Google Scholar]
- 39.Gruener NH, Lechner F, Jung MC, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–5558. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamal SM, Bianchi L, Al Tawil A, et al. Specific cellular immune response and cytokine patterns in patients coinfected with hepatitis C virus and Schistosoma mansoni. J Infect Dis. 2001;184:972–982. doi: 10.1086/323352. [DOI] [PubMed] [Google Scholar]
- 41.Kamal SM, Rasenack JW, Bianchi L, et al. Acute hepatitis C without and with schistosomiasis: correlation with hepatitis C-specific CD4 (+) T-cell and cytokine response. Gastroenterology. 2001;121:646–656. doi: 10.1053/gast.2001.27024. [DOI] [PubMed] [Google Scholar]
- 42.Cerwenka A, Carter LL, Reome JB, Swain SL, Dutton RW. In vivo persistence of CD8 polarized T cell subsets producing type 1 or type 2 cytokines. J Immunol. 1998;161:97–105. [PubMed] [Google Scholar]
- 43.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8 (+) regulatory T cells in vivo in humans. Blood. 2002;100:174–177. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 44.Bukh J, Purcell RH, Miller RH. Sequence analysis of the core gene of 14 hepatitis C virus genotypes. Proc Natl Acad Sci USA. 1994;91:8239–8243. doi: 10.1073/pnas.91.17.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitomi Y, McDonnell WM, Killeen AA, Askari FK. Sequence analysis of the hepatitis C virus (HCV) core gene suggests the core protein as an appropriate target for HCV vaccine strategies. J Viral Hepat. 1995;2:235–241. doi: 10.1111/j.1365-2893.1995.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 46.Soguero C, Joo M, Chianese-Bullock KA, Nguyen DT, Tung K, Hahn YS. Hepatitis C virus core protein leads to immune suppression and liver damage in a transgenic murine model. J Virol. 2002;76:9345–9354. doi: 10.1128/JVI.76.18.9345-9354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao ZQ, Ray S, Eisen-Vandervelde A, Waggoner S, Hahn YS. Hepatitis C virus: immunosuppression by complement regulatory pathway. Viral Immunol. 2001;14:277–295. doi: 10.1089/08828240152716547. [DOI] [PubMed] [Google Scholar]
- 48.Hahn CS, Cho YG, Kang BS, Lester IM, Hahn YS. The hepatitis C virus (HCV) core protein acts as a positive regulator of fast-mediated apoptosis in a human lymphoblastoid T cell line. Virology. 2000;276:127–137. doi: 10.1006/viro.2000.0541. [DOI] [PubMed] [Google Scholar]
- 49.Large MK, Kittlesen DJ, Hahn YS. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J Immunol. 1999;162:931–938. [PubMed] [Google Scholar]
- 50.Woollard DJ, Grakoui A, Shoukry NH, Murthy KK, Campbell KJ, Walker CM. Characterization of hepatitis C virus (HCV)-specific Patr class II restricted CD4+ T cell responses in an acutely infected chimpanzee. Hepatology. 2003;38:1297–1306. doi: 10.1053/jhep.2003.50478. [DOI] [PubMed] [Google Scholar]
- 51.Grakoui A, Shoukry NH, Woollard DJ, et al. Hepatitis C virus (HCV) persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 52.Shoukry NH, Grakoui A, Houghton M, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elrefaei M, El-Sheikh N, Kamal K, Cao H. Hepatitis C virus (HCV)-specific CD27- CD28- memory T cells are depleted in hepatitis C virus and Schistosoma mansoni co-infection. Immunology. 2003;110:513–518. doi: 10.1111/j.1365-2567.2003.01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Estaquier J, Marguerite M, Sahuc F, Bessis N, Auriault C, Ameisen JC. Interleukin-10-mediated T cell apoptosis during the T helper type 2 cytokine response in murine Schistosoma mansoni parasite infection. Eur Cytokine Netw. 1997;8:153–160. [PubMed] [Google Scholar]
- 55.Hesse M, Piccirillo CA, Belkaid Y, et al. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 56.Kamel MA, Miller FD, el Masry AG, et al. The epidemiology of Schistosoma mansoni, hepatitis B and hepatitis C infection in Egypt. Ann Trop Med Parasitol. 1994;88:501–509. doi: 10.1080/00034983.1994.11812897. [DOI] [PubMed] [Google Scholar]
- 57.Chensue SW, Wellhausen SR, Boros DL. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981;127:363–367. [PubMed] [Google Scholar]


