Fig. 4. Putative substrate-binding sites.
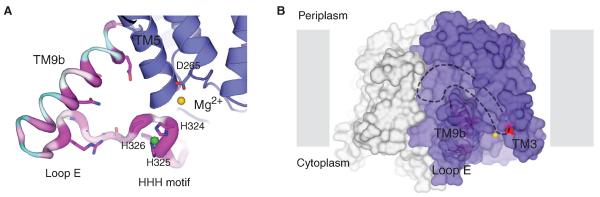
(A) Zoomed-in view of TM9b, loop E (the HHH motif). The structure is rotated 90° toward the reader relative to Fig. 3A. TM9b/loop E is shown in sausage representation, with the thicker region more conserved (magenta) and the thinner region less conserved (cyan). Side chain of highly conserved amino acids on TM9b and loop E as well as the catalytic residue D265 are shown in stick representation. Mg2+ and Ni2+ are shown as yellow and green spheres, respectively. (B) A hydrophobic groove connected to the active site is delineated with dashed lines on the MraYAA surface illustration. Mg2+ is shown as a yellow sphere, and D117 is colored red. Protomers are colored blue and gray.
