Fig. 8.
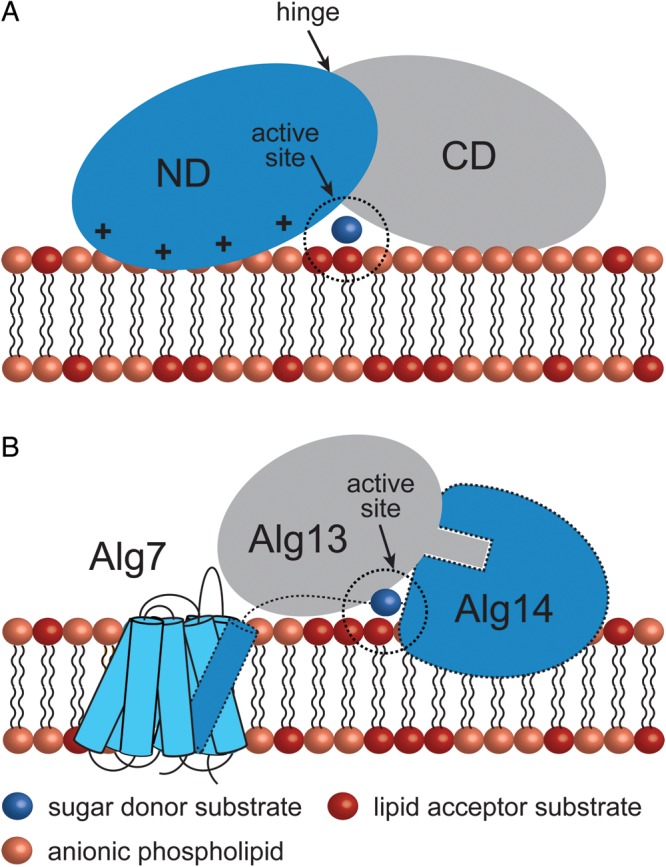
Peripheral GT-B GTs adopt different strategies for membrane association. (A) The mycobacterial mannosyltransferase PimA interacts with the phospholipid bilayer by a combination of positively charged and hydrophobic residues exposed on the N-terminal domain of the protein. An important conformational change is expected to occur during/after protein–membrane interaction (Guerin et al. 2007; Guerin et al. 2009; Giganti et al. 2013). (B) A model for the Alg7/13/14 complex formation. This functional multienzyme complex catalyzes the first two steps of lipid-linked oligosaccharide on the cytosolic face of the ER membrane. Alg7, a politopic protein, which transfers a GlcNAc-phosphate to dolichol phosphate, interacts with the transmembrane α-helix of the bitopic GT-GT Alg14 (Lu et al. 2012). Biochemical and structural data support a model in which Alg13-Ag14 interaction is mediated by residues located on the C-terminal α-helix of Alg13 and C-terminal amino acids of Alg14 to form a dimeric Alg13/14 (Wang et al. 2008).
