Figure. Residues in motion.
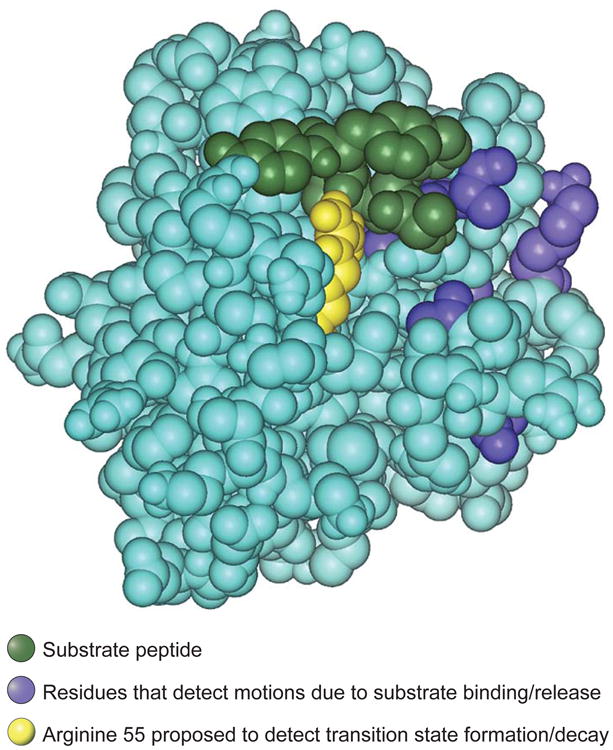
Space-filling atomic structure of the complex between the enzyme cyclophilin A and its substrate cis-Suc-Ala-Phe-Pro-4-NA (20). The substrate is green; residues whose backbone amides detect motional changes due to repetitive substrate binding and dissociation are blue. The catalytic residue arginine 55 is shown in yellow. This residue forms a hydrogen bond to the substrate and detects a motional frequency similar to the catalytic rate, suggesting that it could be involved in motions leading to the formation and decay of the transition state.
