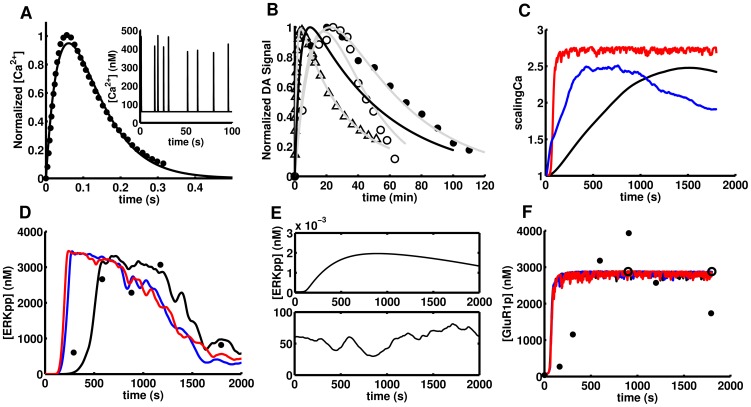
Figure 4. Inputs and outputs in the acute psychostimulant administration paradigm.
A) A synaptically evoked Ca2+ spike in a single dendritic spine as measured with a fluorescent Ca2+ indicator [88]. Inset: 100 seconds of simulated random Ca2+ spikes. B) Different measurements of psychostimulant induced DA overflow in the striatum: C11-cocaine levels in the brain after i.v. administration in humans (Δ), the DA mediated psychostimulant effects follows the same kinetics [89]; nomifensine-evoked DA measured by FSCV [90] (○) and amplitude of electrically evoked DA by FSCV after cocaine administration (•). Eq.7 fit these three experimental datasets with r2>0.96 in all cases (solid grey). The solid black curve is the psychostimulant-induced DA overflow used in this work. C) Time course of the scaling factor for the three NMDAR enhancement mechanisms upon APA: the single channel mechanisms via serine/threonine (red) or tyrosine phosphorylation (blue) and the traffic based mechanism (black). D) Just the traffic-based mechanism of scaling fits the ERK activation data (r2 = 0.87). E) ERK is not significantly activated either by DA in the absence of Ca2+ spikes (upper panel) or Ca2+ spikes with no dopamine increase (lower panel) showing its capability as an AND gate. F) Simulated phosphorylation of GluR1 by PKA and two experimental data sets from APA-treated mice, one for methamphetamine (10 mg/Kg, •) [12] and the other for cocaine (20 mg/Kg, ○) [13]. The time course of GluR1 phosphorylation shows no dependence on the scaling mechanism. The time course of GluR1 and ERK phosphorylation was simulated with a single random Ca2+ spike train.