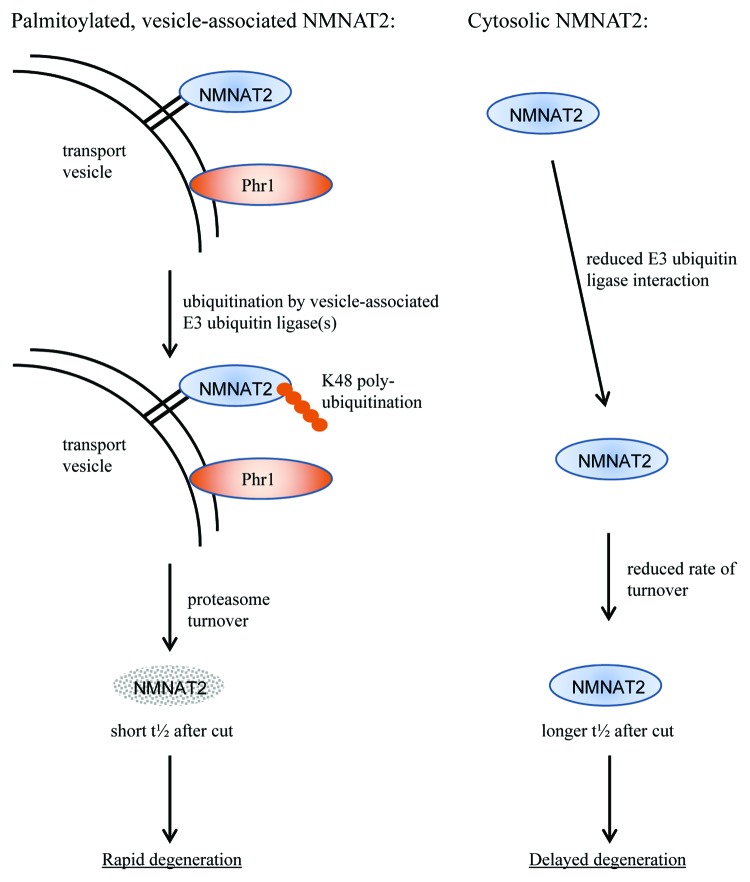
Figure 2. Model of the mechanism of vesicle association–induced ubiquitination and turnover of NMNAT2. Palmitoylation promotes membrane association of NMNAT2, thus bringing it into close proximity with Phr1 and/or other putative E3 ubiquitin ligases. The close association and confinement to a 2D space strongly promote NMNAT2 K48-linked poly-ubiquitination and rapid turnover by the proteasome. Thus, NMNAT2 half-life is short, resulting in rapid depletion after axotomy or interruption of axonal transport and, ultimately, rapid axon degeneration. In contrast, cytosolic NMNAT2 encounters relevant E3 ubiquitin ligases much less frequently, resulting in lower levels of K48-linked poly-ubiquitination and a slower rate of UPS-mediated turnover. Consequently, the half-life of cytosolic NMNAT2 is prolonged, resulting in a longer latent phase and delayed axon degeneration.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.