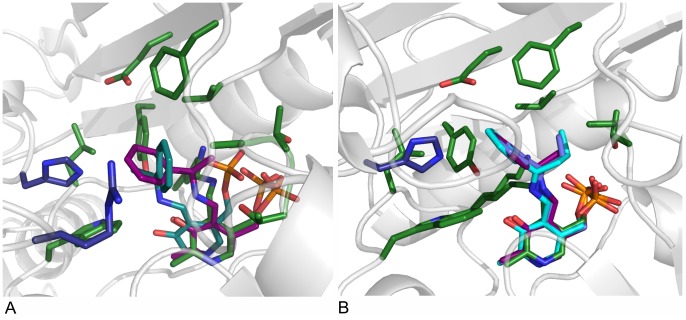
Figure 4. Docking of various substrate intermediates into AT-ωTA.
A: Pro-(R)- and (S)-acetophenone pyridoxal phosphate intermediates docked into the active site of AT-ωTA. Green: amino acids of the active site (chain A) and PLP bound to K180, blue: amino acids of the active site (chain B), purple: pro-(R)-acetophenone pyridoxal phosphate intermediate, turquoise: pro-(S)-acetophenone pyridoxal phosphate intermediate. B: Acetophenone pyridoxal phosphate intermediate (purple), propiophenone pyridoxal phosphate intermediate (blue), butyrophenone pyridoxal phosphate intermediate (turquoise) docked into the active site of AT-ωTA compared to PLP bound to K180 in the structure of AT-ωTA (green). Green: amino acids of the active site (chain A), blue: amino acids of the active site (chain B). The figures were prepared using the program PyMOL.