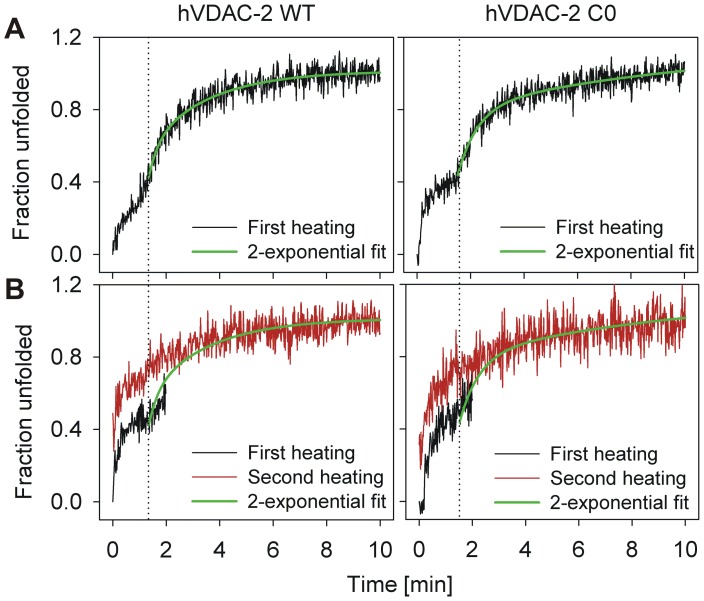
Figure 5. hVDAC-2 unfolding is irreversible, despite the presence of an unfolding intermediate.
(A) 5 µM of refolded protein in 65 mM LDAO was subjected to thermal denaturation at 80°C, and the loss in ME215 (shown here after conversion to fraction unfolded) was recorded. Two transitions were seen in the case of both WT and C0, with the second transition starting at ∼2 min. (B) To check whether the first transition was due to reversible or irreversible unfolding, a fresh batch of refolded protein in 65 mM LDAO was subjected to 80°C for 2 min (black traces), after which the cuvette was immediately transferred to 4°C for 10 min before re-heating at 80°C (red traces). Visible aggregation was not observed after the initial 2 min heating. The second (red) trace, however, did not show the two transitions expected for this experimental condition, and instead only exhibited a single transition corresponding to protein aggregation. This indicates that the first transition is likely to arise from an irreversible change in protein conformation that does not correspond to the aggregated species. Shown in green are the fits of the second transition seen in (A) to an exponential function. The same fits are also depicted in (B) to highlight the difference in the profile of the re-heated samples (red trace) in the first 2 min of the recording, as well as demonstrate that similar end-points are achieved in both experiments.