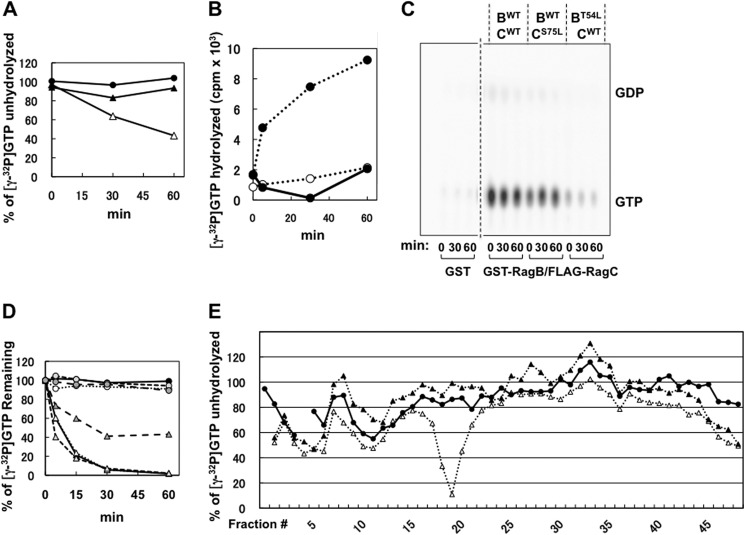
FIGURE 3.
Rag heterodimers lack detectable GTPase activity in vitro. A, the RagB/C wild-type heterodimer does not hydrolyze [γ-32P]GTP in vitro. GST-Ha-RasWT (▵), GST-Ha-RasG12V (▴), and GST-RagBWT/FLAG-RagCWT (●) were charged with [γ-32P]GTP and examined for intrinsic GTPase activity. The amount of unhydrolysed [γ-32P]GTP on proteins was measured at intervals thereafter by nitrocellulose filter binding assay. B, a recombinant catalytic fragment of NF1 stimulates GTP hydrolysis by Ha-RasWT but not Ha-RasG12V or RagBWT/CWT. GST-Ha-RasWT (●, dashed line), GST-Ha-RasG12V (○, dashed line), or GST-RagBWT/FLAG-RagCWT (●, solid line) were charged with [γ-32P]GTP, and a catalytic fragment of NF1 was added. The release of 32Pi at 30 °C was measured at intervals thereafter by the charcoal method. C, neither RagB wild-type or RagC wild-type within a heterodimer hydrolyze [α-32P]GTP detectably in vitro. GST and various GST-RagB/FLAG-RagC heterodimers were charged with [α-32P]GTP, and at intervals thereafter, nucleotides were extracted and separated on TLC on PEI cellulose. D, extracts from HEK293T cells do not promote the ability of RagB/C to hydrolyze [γ-32P]GTP. HEK293T cells were lysed in the absence of detergent (solid line) or with CHAPS (0.3%, dotted line), TritonX-100 (1%, short dashes), or RIPA buffer (Pierce) (long dashes). GST-Ha-RasWT (triangles) and GST-RagBWT/FLAG-RagCWT (circles) charged with [γ-32P]GTP were mixed with each of these extracts at 30 °C, and the amount of protein-bound [γ-32P]GTP was measured by filtration through nitrocellulose filters. E, MonoQ anion exchange chromatography of a HEK293T cell extract contains GAP activity toward Ha-Ras wild-type, not RagB/C wild-type. HEK293T cells were extracted by freezing and thawing and separated by anion exchange chromatography. Each fraction was incubated at 30 °C with GST-Ha-RasWT (▵, dashed lines), GST-Ha-RasG12V (▴, dashed lines), and GST-RagBWT/FLAG-RagCWT, each charged with [γ-32P]GTP. After 30 min, protein-bound [γ-32P]GTP was measured as in C.