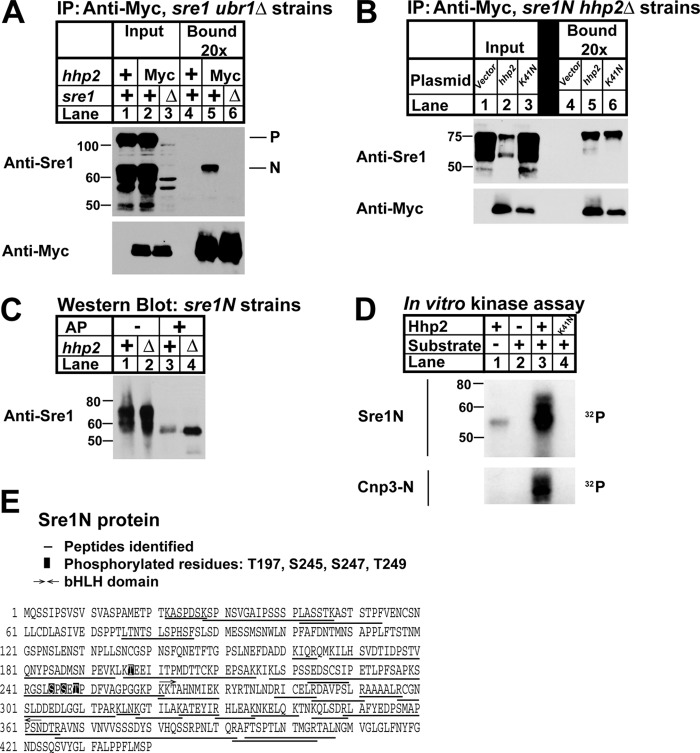
FIGURE 6.
Hhp2 binds and phosphorylates Sre1N. A, co-immunoprecipitation (IP) of Hhp2-Myc and Sre1 from ubr1Δ, ubr1Δ hhp2-myc, and ubr1Δ hhp2-myc sre1Δ cells treated with 2 mm DSP cross-linker in PBS for 30 min was carried out using anti-Myc antibody and Nonidet P-40-solubilized whole-cell lysates. Input and bound (20× overloaded) fractions were analyzed by Western blot using anti-Myc and anti-Sre1. Blots are representative of three independent experiments. P and N denote the precursor and nuclear forms of Sre1, respectively. B, co-immunoprecipitation of Hhp2 and Sre1N in sre1N hhp2Δ cells carrying the indicated plasmids was carried out as in A except cells were grown for 4 h in the absence of oxygen, switched to normoxic conditions, and treated with 2 mm DSP cross-linker in PBS for 30 min prior to lysis. Anti-Myc bound is 10× overloaded. Blots are representative of three independent experiments. C, Western blot of whole-cell lysates from sre1N and sre1N hhp2Δ cells grown in the presence of oxygen and treated with 1 mm bortezomib (BZ) for 3 h. Lysates in lanes 3 and 4 were treated with alkaline phosphatase (AP). sre1N samples (lanes 1 and 3) were 12× overloaded relative to mutant samples to enable visualization of Sre1N protein. D, in vitro kinase assay was performed with the indicated purified recombinant Hhp2 and either Sre1N or Cnp3-N as substrate. Kinase reaction products were resolved by SDS-PAGE, and autoradiograms are shown. E, sequence of Sre1N showing mass spectrometry results of Sre1N-Hhp2 in vitro kinase reaction. Peptides identified are underlined. Phosphorylated residues are boxed.