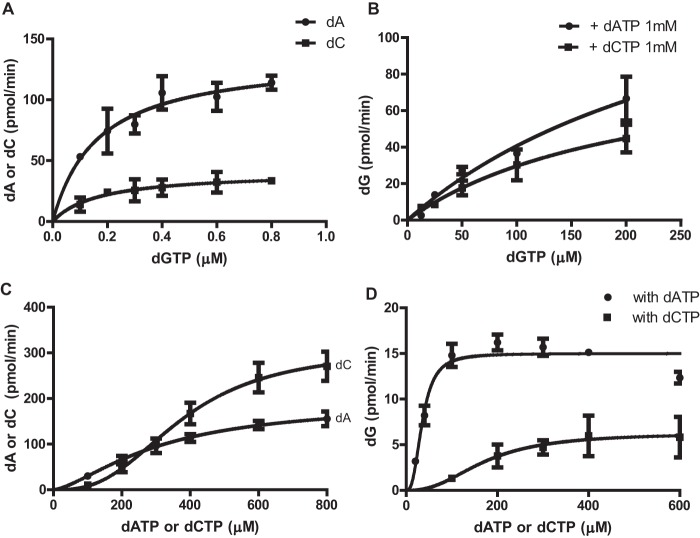
FIGURE 5.
Enzyme kinetics of dGTP-, dATP-, and dCTP-dependent dNTPase activity of EF1143. A, the rate of dATP (circles) or dCTP (squares) conversion was determined in the presence of dGTP (0–0.8 μm), with an initial dATP or dCTP concentration of 1 mm. The data were fitted with the Michaelis-Menten model (Equation 1). Vmax and Km were determined to be 47.0 ± 3.4 nmol/nmol·min and 0.17 ± 0.05 μm, respectively, for dATP, and 11.5 ± 1.7 nmol/nmol·min and 0.17 ± 0.08 μm, respectively, for dCTP. B, the rate of dGTP conversion was determined with the initial dGTP concentration ranging from 0 to 200 μm and a fixed concentration (1 mm) of dATP (circles) or dCTP (squares). Vmax and Km were determined to be 47.3 ± 17.8 nmol/nmol·min and 315 ± 170 μm, respectively, for the dGTP/dATP mixture, and 25.9 ± 7.3 nmol/nmol·min and 213 ± 98 μm, respectively, for the dGTP/dCTP mixture when the data were fitted with the Michaelis-Menten model (Equation 1). C, the rate of dATP (circles) or dCTP (squares) conversion was determined in the presence of increasing concentrations of dATP (circles) or dCTP (squares) and a fixed concentration of dGTP (16 μm). The data were fitted with an allosteric sigmoidal model (Equation 2). Vmax, Khalf, and Hill coefficient (h) were determined to be 54.1 ± 7.0 nmol/nmol·min, 310 ± 63 μm, and 1.5 ± 0.3, respectively, for dATP. The kinetic values for dCTP were 86.2 ± 7.8 nmol/nmol·min, 376 ± 36 μm, and 2.7 ± 0.5, respectively. D, the rate of dG production was also determined from the experiments performed in C, and the data were fitted with an allosteric sigmoidal model. Vmax, Khalf, and h for dGTPase activity in the presence of dATP (circles) were determined to be 4.2 ± 0.1 nmol/nmol·min, 35 ± 4 μm, and 2.7 ± 0.7, respectively. In the presence of varying dCTP concentrations (squares), the kinetic values for dGTPase activity were 1.8 ± 0.3 nmol/nmol·min, 172 ± 46 μm, and 2.4 ± 1.4, respectively. Error bars, S.D.