FIGURE 2.
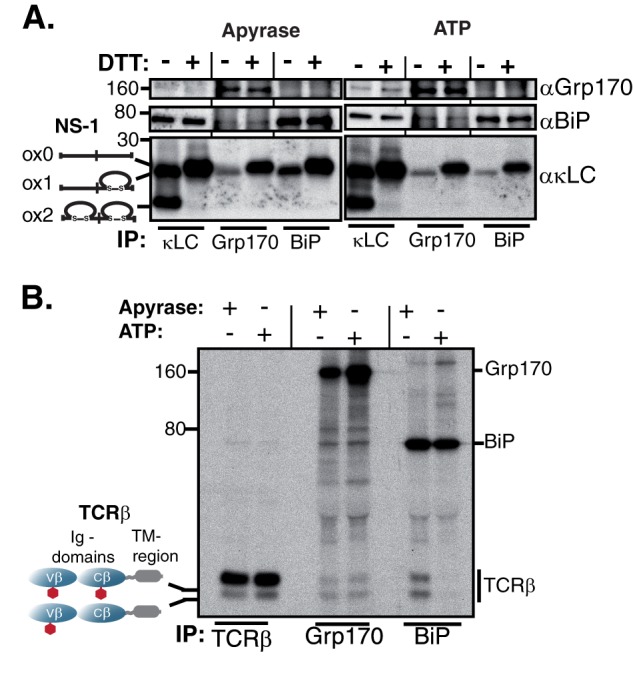
Grp170 and BiP bind to the same molecular species of Ig substrates. A, unlabeled P3U.1 murine myeloma cells were lysed either in the presence of apyrase or ATP, immunoprecipitated with the indicated reagents and analyzed under non-reducing conditions. As BiP is ∼10-fold more abundant than Grp170 in the cell (46), five times more lysate was used for Grp170 immunoprecipitations than for κLC or BiP. Isolated proteins were separated on 13% SDS-PAGE gels and transferred for blotting with the indicated reagents. B, COS-1 cells were transfected with vectors encoding TCRβ, Grp170, and BiP, pulse-labeled with [35S]cysteine/methionine for 0.5 h, chased for 1 h and lysed either in the presence of apyrase or ATP. Interactions between TCRβ, Grp170, and BiP were analyzed via immunoprecipitation with the indicated antisera and separation on 10% SDS gels. Four times less lysate was used for the TCRβ immunoprecipitation than for Grp170 or BiP to make both species of TCRβ visible. The mobility of the two TCRβ glycoforms is indicated.
