FIGURE 1.
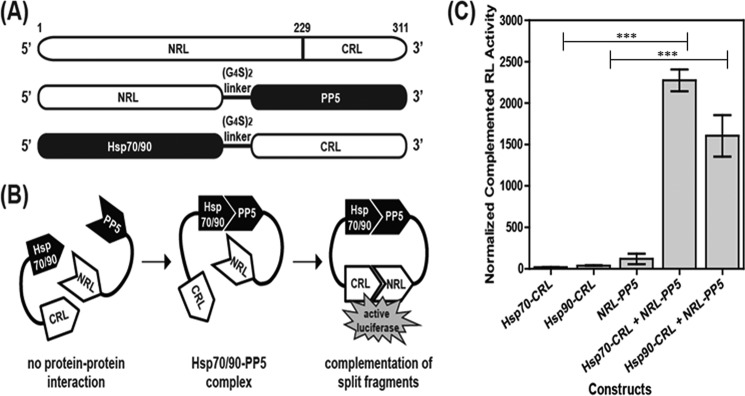
SRL-PFACs confirm that both Hsp70-PP5 and Hsp90-PP5 interact. A, schematic diagram of plasmid constructs. The two interacting proteins PP5 and Hsp70/90 are fused to NRL (amino acids 1–229) and CRL (amino acids 230–311) portion of the RL, respectively, through a (G4S)2 peptide linker. B, schematic diagram of the SRL-PFAC system for monitoring complex formation between Hsp70/90 and PP5. Interactions between Hsp70/90 and PP5 bring CRL and NRL in close proximity, ultimately resulting in the complementation of RL enzyme activity and photon production in the presence of the substrate coelenterazine. C, SRL-PFAC system is sensitive for monitoring complex formation of Hsp70/90 and PP5 and shows highly complemented RL activity and low background. HEK293 cells were transduced with either Hsp70-CRL, Hsp90-CRL, NRL-PP5, Hsp70-CRL+NRL-PP5, or Hsp90-CRL+NRL-PP5. Data are presented as mean ± S.D. (n = 3). ***, p < 0.001 compared with controls.
