FIGURE 3.
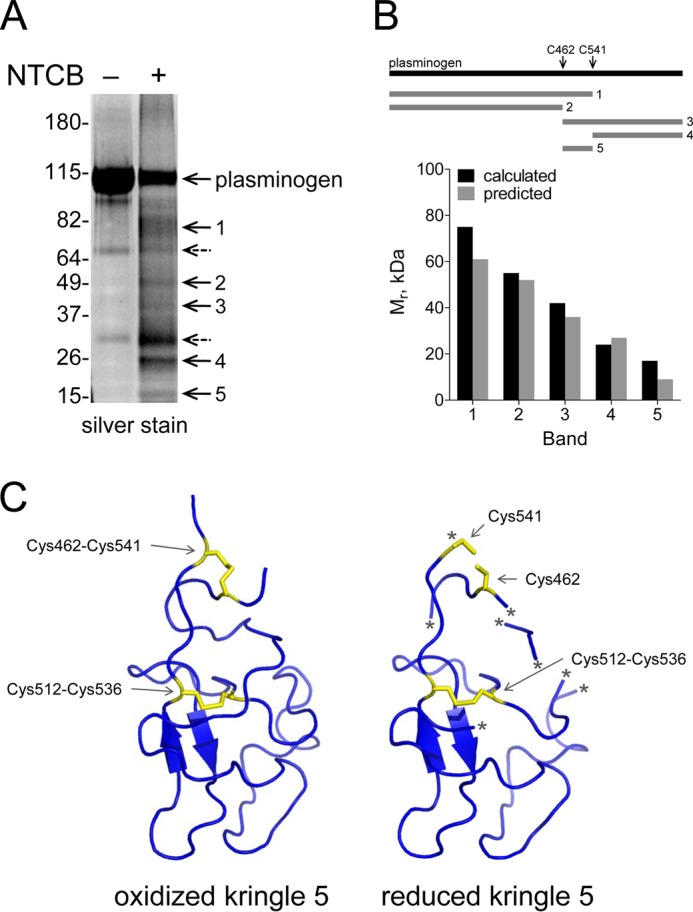
The Cys462-Cys541 disulfide bond is reduced in a fraction of plasminogen. A, plasminogen in pH 8.0 buffer was untreated or reacted with NTCB. The peptide bond N-terminal of the cyanylated cysteine was then cleaved at pH 9. The fragments were reduced and alkylated, resolved by SDS-PAGE, and stained with SYPRO Ruby. Five distinct fragments were generated (bands 1–5). Two nonspecific fragments that were present in the untreated plasminogen are indicated by dotted arrows. The positions of molecular mass markers are shown at left. B, the molecular masses of the five fragments are consistent with NTCB cleavage of a fraction of plasminogen containing unpaired cysteines at residues 462 and 541. Comparison of the measured and predicted fragment sizes based on NTCB cleavage at cysteines 462 and 541 is shown in the bar graph. The identity of the fragments was confirmed by mass spectrometry (Table 2). C, structure of plasminogen kringle 5 containing a reduced Cys462-Cys541 disulfide bond. Ribbon structure of kringle 5 (residues 460–543) of full-length human plasminogen shows the Cys462-Cys541 and Cys512-Cys536 disulfide bonds in yellow. The Cys462-Cys541 disulfide bond is reduced in the structure at right. The asterisk represents regions of the polypeptide chain of the reduced structure that were not resolved by x-ray crystallography. The structure is that of PDB identifier 4DUR (28).
