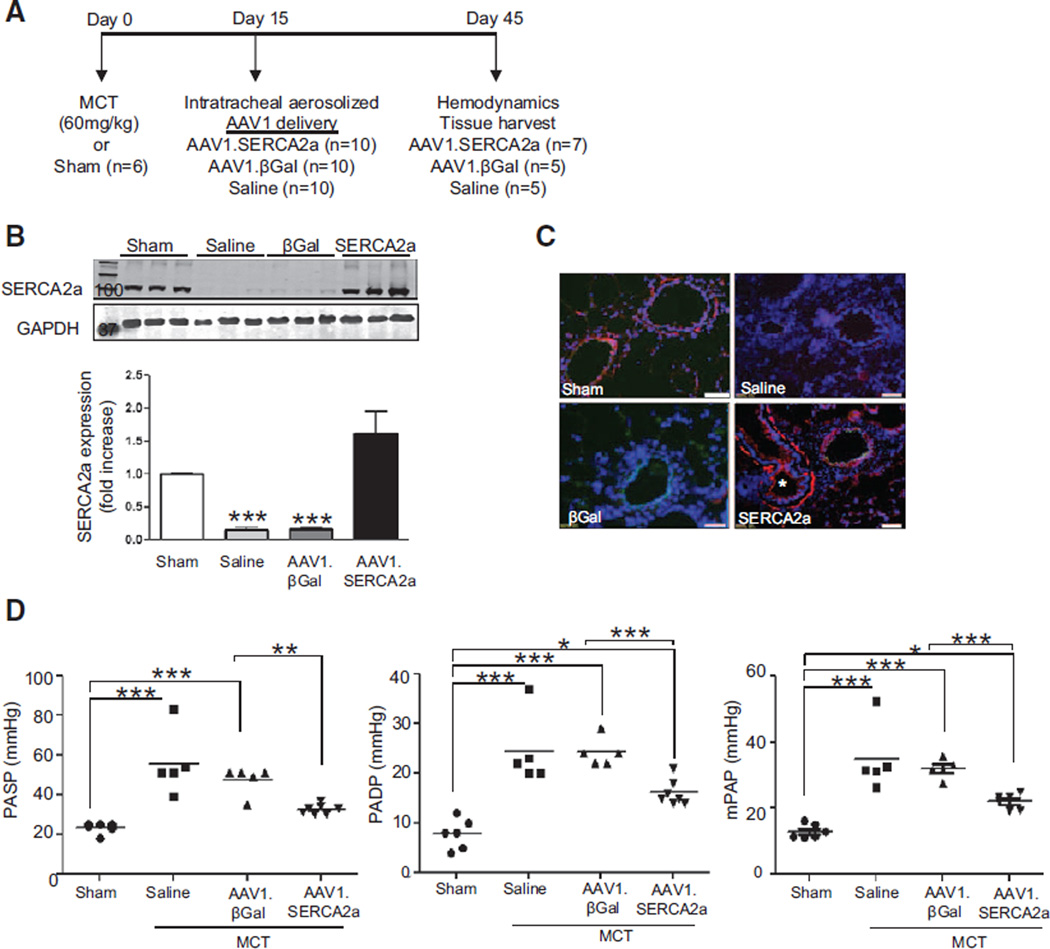
Figure 3.
Intratracheal delivery of aerosolized AAV1.SERCA2a improves pulmonary hemodynamics in the rat MCT model of PAH. A, Schematic of the treatment protocol to assess the therapeutic efficacy of AAV1.SERCA2a gene therapy when given after PAH is established. B, SERCA2a protein expression in lung homogenates from the sham (n=6) and MCT-PAH rats treated with aerosolized saline (n=5), AAV1.βGal (n=5), or AAV1.SERCA2a (n=7) was examined by Western blot. GAPDH was used as a loading control. A representative blot (n=3) is shown. ***P<0.001 vs saline, AAV1.SERCA2a. C, SERCA2a expression (red) in pulmonary arterioles was detected by immunofluorescence. Nuclei were counterstained with DAPI (blue). * (lower right) refers to airway with visible SERCA2a expression in the smooth muscle layer. Scale bar, 100 µm. D, The effect of AAV1.SERCA2a gene therapy on pulmonary artery systolic pressure (PASP), diastolic pressure (PADP), and mean pulmonary pressure (mPAP) was measured invasively at day 45. *P<0.04 vs sham or AAV1.βGal, **P<0.004 vs AAV1.βGal, ***P<0.001 vs sham or AAV1.βGal. DAPI indicates 4',6-diamidino-2-phenylindole; MCT, monocrotaline; and PAH, pulmonary arterial hypertension.