Abstract
Objective:
To examine how cognitive impairment affects Parkinson disease (PD) patients' research consent capacity.
Methods:
A cross-sectional study of 90 patients with PD, divided using Mattis Dementia Rating Scale–2 scores into 3 groups of 30 (normal, borderline, and impaired), and 30 neurologically normal older adults completed 2 capacity interviews (an early-phase randomized and controlled drug trial and a sham-controlled surgical implantation of genetic tissue) using the MacArthur Competence Assessment Tool for Clinical Research. Expert clinicians used the interviews to classify the patients as either capable or not capable of providing their own informed consent. These judgments were compared with performance on the Montreal Cognitive Assessment (MoCA) and the Mini-Mental State Examination (MMSE).
Results:
Cognitively normal PD patients typically scored well on the capacity measures. In contrast, patients with impaired cognition were not capable of providing their own informed consent: 17% (5/30) on the drug trial and 3% (1/30) on the surgery trial were judged capable. Patients with borderline impairment showed adequate performance on measures of appreciation and reasoning, but impaired performance on understanding the drug trial compared with normal controls and normal PD patients, and on understanding the surgery trial compared with normal controls. Sixty-seven percent (20/30) on the drug trial and 57% (17/30) on the surgery trial were judged capable of consent. Receiver operating characteristic analyses showed that the MMSE and MoCA could detect the likelihood of impaired capacity, with the MoCA demonstrating greater sensitivity.
Conclusions:
PD patients with borderline cognitive impairment have impairments in their decisional capacity. The MoCA may be useful to identify the patients at risk of impaired capacity.
Parkinson disease (PD) leads to functional impairments as a result of not only motor, but also cognitive deficits.1 Even patients with mild cognitive impairment (MCI) have impairments in their function,2 and their capacity to make medical decisions,3–5 but as prevalent and clinically significant as cognitive impairment is, little is known about how it affects patients' research consent capacity.
Researchers need to know which decisional abilities are impaired, and how these impairments associate with measures of overall cognition such as the Montreal Cognitive Assessment (MoCA) and the Mini-Mental State Examination (MMSE). These measures are often among the clinical trial eligibility criteria, and clinicians use them to measure cognitive impairment in PD. Although cognitive measures cannot substitute for a capacity assessment, the more we understand how cognitive impairments associate with losses in capacity, the better we can delineate the borders between normal vs abnormal cognition, and have an evidence base to develop protocol-specific subject protections.
Studies of medical decision-making capacity in patients with dementia and MCI use a similar conceptual model as studies of research consent capacity.3–5 These studies suggest that we should expect impairments in research consent capacity. The goals of this study were to understand the severity of capacity impairments in a cohort of patients with PD whose overall cognitive performance ranged from normal to dementia-range impairment. Our hypotheses were that PD patients in the borderline range of impairment would, compared with cognitively normal PD patients, show impairments in their decisional abilities, and that the MoCA and the MMSE would associate with a PD patient's loss of capacity to provide informed consent.
METHODS
Study participants and eligibility criteria.
We used a nonproportional stratified sampling process to enroll 90 patients with PD and divide them into 3 groups of 30 patients each using cutoffs based on Mattis Dementia Rating Scale–2 (DRS-2) performance (PD patients with “normal,” “borderline,” and “impaired” cognition). We also enrolled 30 neurologically normal older adults as a normative reference for all 3 patient groups' capacity scores (“normal controls”). All subjects were aged 65 years or older, native English speakers with at least sixth grade education, had a corrected visual acuity to read from a handheld visual acuity card, and were able to hear spoken speech.
Patients with PD.
Patients with PD were eligible if they had 1) a diagnosis of idiopathic PD by a movement disorder neurologist at the Penn Udall Center, 2) the ability to give consent, or assent with the consent of a legally authorized representative, and 3) performance on the DRS-2 total age- and education-corrected MOANS (Mayo Older Americans Normative Studies) Scaled Score in one of the following 3 cognitive categories: “normal” DRS-2 ≥9, “borderline” DRS-2 = 6 to 8, and “impaired” DRS-2 ≤5. We selected these categories based on the published norms of the DRS-2 and evidence that these categories differentiate among patient performance on a measure of instrumental activities of daily living.2,6 All PD patients were interviewed “on” medication, as has been the practice in other studies of capacity and cognition.
Normal controls.
Persons aged 65 years or older enrolled in the normal control cohort of the Penn Alzheimer's Disease Center longitudinal cohort study were interviewed within 3 months of their last cohort assessment and defined as having normal age- and education-adjusted performance on neuropsychological testing, a normal neurologic examination, no diagnosis of idiopathic PD, and lacking both dementia by NINCDS-ADRD (National Institute of Neurological and Communicative Diseases and Stroke–Alzheimer's Disease and Related Disorders Association) criteria7 and MCI by Peterson criteria.8
Subject measures.
Two research assistants divided data collection into 2 days in order to limit participant fatigue and to ensure that the capacity interviewer was blinded to cognitive data. Subjects underwent 2 MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR) interviews, one describing an early-phase, double-blind, placebo-controlled “bridging study” to test the safety and tolerability of a drug, a capacity scenario we have used in prior research,9,10 and the other describing a double-blind, sham-surgery controlled, randomized trial to test the safety and tolerability of injecting a growth factor gene into the brains of patients with PD.
We chose these 2 clinical trials because they involved the spectrum of research procedures currently being tested in patients with PD, and as early-phase trials with more than minimal risks, they require additional subject protections such as a capacity assessment. Also, 2 clinical trials allowed us to examine the consistency of patient performance between trials and, because we expected normal controls to demonstrate relatively sound decision-making capacity on the MacCAT-CR, 2 sets of capacity scores would permit a more robust estimate of the range of normal performance.
For each capacity interview, subjects retained a copy of that clinical trial's mock informed consent form to consult at their discretion while answering the MacCAT-CR interview questions. The forms were written in the style and format of Penn Institutional Review Board–approved informed consent forms and included the content mandated under the requirements for informed consent described in the Common Rule.11
The assessment of decision-making capacity.
To assess capacity after the disclosure session, we used the MacCAT-CR,12 a standardized assessment that measures the 4 decision-making abilities (score ranges in parentheses): understanding (0–26), appreciation (0–6), reasoning (0–8), and expressing a choice (0–2). Its reliability and validity has been shown in persons with major depression,13 schizophrenia,14,15 and mild to early moderate Alzheimer disease.16,17 The interviewer scored the interview as she performed it and also digitally video recorded the interview for quality control, review, and rating by expert judges.
The standard MacCAT-CR administration was modified to more accurately reflect the way informed consent is conducted in standard research practice. Instead of asking an understanding question after each section, the forms were reviewed in their entirety before asking the MacCAT-CR questions. At the start of the capacity assessment, the interviewer encouraged the subject to refer to the informed consent form while he or she answered the questions.
The judgment that a patient is capable of informed consent.
Three psychiatrists located outside of Penn, with at least 5 years of postresidency expertise in capacity assessment and familiar with the MacCAT-CR conceptual model for decisional capacity, independently viewed each capacity interview and rated whether the subject was capable of providing his or her own informed consent. Expert raters answered the following question that we and other researchers have employed: “Based on your review of this interview, do you believe that this subject has sufficient capacity to give his or her own informed consent to the research study?” with answer choices of “definitely has sufficient capacity,” “probably has sufficient capacity,” “probably does not have sufficient capacity,” and “definitely does not have sufficient capacity.”9,17–19
The status of capable of consent was defined by the consensus of at least 2 of 3 experts. Consensus of expert rater outcomes have generally shown good overall agreement.16,19–22 To ensure that the experts focused only on the capacity data and that performance on measures of cognition did not influence their capacity judgments, raters were blinded to all clinical data that characterized cognitive, functional, and PD symptom-related severity.
Covariates of subject demographics, disease severity, and cognition.
Covariates included age, education in years, sex, race, and ethnicity; Hoehn and Yahr stage23; levodopa equivalent daily dose24; Geriatric Depression Scale–1525; MMSE26; MoCA27; and DRS-2 total scores.28
Data analyses.
All analyses were performed using STATA 12.0 (StataCorp, College Station, TX). As expected, normal control performances on each of the MacCAT-CR interviews did not differ (paired t tests: understanding, p = 1.00; appreciation, p = 0.77; reasoning, p = 0.42), allowing us to sum each pair of ability scores, rescale them according to the range of the ability measure, and use this distribution to categorize PD patient performance.
The cutoff was set at ≥15.9th percentile, which corresponds to 1 SD below the mean of a normal distribution. We calculated the proportions of PD patients in each of the cognitive groups who had intact performance and compared these proportions using the χ2 test. Similar comparisons were made among the proportions judged capable of informed consent.
To examine the relationship between the judgment that a patient with PD is capable of informed consent and measures of overall cognition, we used logistic regression and STATA's “roctab” function to examine the sensitivity and specificity of being judged not capable of providing informed consent, to generate receiver operating characteristic (ROC) curves, and to compute the area under the ROC curve (AUC) and its 95% confidence interval (CI); and the “roccomp” function to determine whether there is one ability measure that performs better than the rest.
Standard protocol approvals, registrations, and patient consents.
All participants provided written informed consent, or in the case of those not capable, assent with the informed consent of their knowledgeable informant, to participate in this University of Pennsylvania Institutional Review Board–approved study.
RESULTS
Subject characteristics.
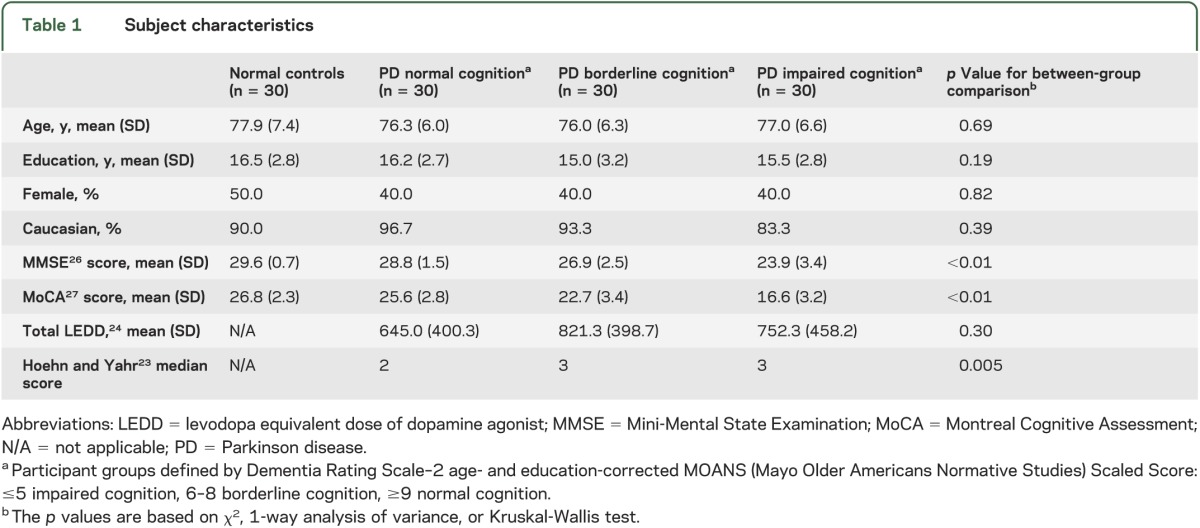
Table 1 shows that the 4 subject groups with 30 subjects each—normal controls and PD patients with normal, borderline, or impaired cognition—had no differences in their age, years of education, sex, or race. PD patients with normal cognition had a higher median Hoehn and Yahr score than borderline and impaired PD patients.
Table 1.
Subject characteristics
Performance on decisional capacity.
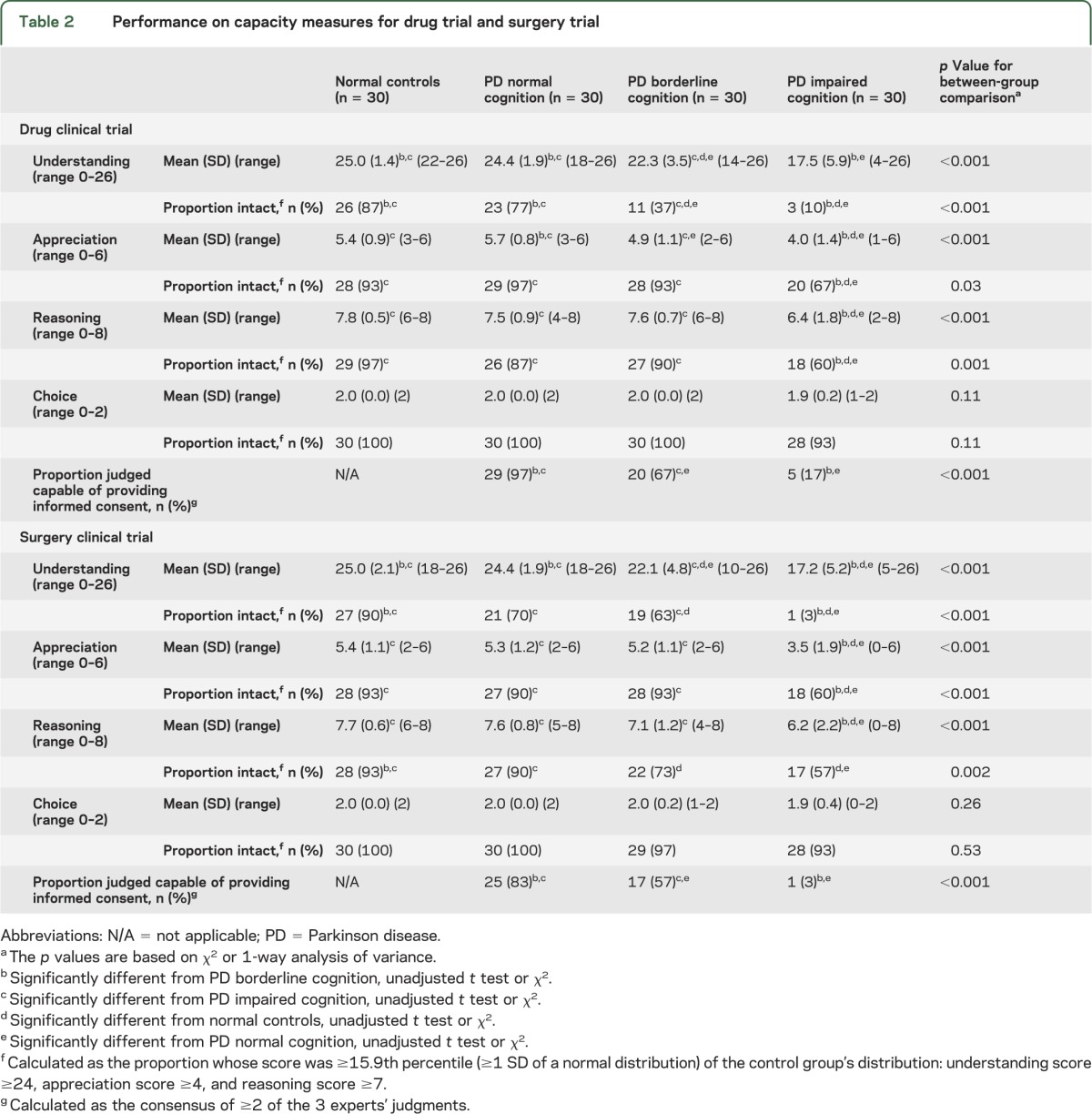
Table 2 summarizes the mean scores, proportion intact on the measures of decision-making ability as defined by the performance of the normal controls, and the consensus of the 3 independent experts' judgments of the PD patients' ability to provide their own informed consent. The judgments of the 3 independent experts showed good agreement for both clinical trials: pairwise κ values ranged from 0.56 to 0.74, and group κ values of 0.64 for the drug trial and 0.65 for the surgical trial. In general, between-group comparisons of either mean scores or the proportions with intact performance show similar patterns, and, as expected, all subjects performed well on the simplest of abilities, the ability to express a choice. Below, we focus on each PD patient group's proportion intact on understanding, appreciation, and reasoning, and the expert judgments, because they summarize capacity performance in a more clinically transparent manner.
Table 2.
Performance on capacity measures for drug trial and surgery trial
PD patients with normal cognition.
PD patients with normal cognition compared with normal controls showed generally good performance on all decisional abilities for both clinical trials. The proportions of these patients scoring as well as normal controls ranged from 70% to 100%. The lower end of performance was seen on understanding both the drug and the surgical trials: 70% (21/30) intact on the surgical trial and 77% (23/30) on the drug trial. Most of these patients were judged capable of providing their own informed consent for the drug and the surgery trials (97% and 83%, respectively).
PD patients with borderline cognition.
PD patients with borderline cognition had a mixed performance. Compared with normal controls, they had impaired performance on their ability to understand the drug and surgery trials: 37% (11/30) and 63% (19/30), respectively. In contrast, they generally showed adequate performance on measures of appreciation and reasoning, ranging between 90% (27/30) and 93% (28/30), with the exception of their performance on the ability to reason through the surgery trial whereby 73% (22/30) performed adequately. This mixed performance is reflected in the expert judgments of capacity: 67% (20/30) were judged capable of informed consent on the drug trial and 57% (17/30) on the surgery trial.
PD patients with impaired cognition.
PD patients with impaired cognition had notable decisional ability deficits, particularly the ability to understand. Only 10% (3/30) and 3% (1/30) adequately understood the drug and surgery trials, respectively, performance that was worse than both cognitively normal and borderline PD patients. Cognitively impaired PD patients also had significantly worse performance than the other 2 patient groups on measures of appreciation and reasoning. For these abilities, the proportion of cognitively impaired PD patients who scored as well as the normal controls ranged from 57% (17/30) to 67% (20/30). The severity of these capacity impairments was reflected in the expert judgments: only 17% (5/30) were judged capable of informed consent on the drug trial and 3% (1/30) on the surgery trial.
Relationship between expert capacity ratings and patient cognitive impairment.
ROC analyses can demonstrate how each score on a measure of cognition predicts the likelihood that experts judge a patient to have adequate capacity. For the MMSE, the AUC for the drug trial was 0.83 (95% CI: 0.74–0.92) and for the surgery trial was 0.85 (95% CI: 0.77–0.92). For the MoCA, the AUC for the drug trial was 0.88 (95% CI: 0.80–0.96) and for the surgery trial was 0.91 (95% CI: 0.84–0.98). The MoCA showed a trend toward better prediction than the MMSE for the surgery trial (p = 0.06) and no difference for the drug trial (p = 0.22).
Table 3 shows the sensitivity and specificity of being judged not capable of consent for both the drug and surgery trials as a function of performance on the MoCA and the MMSE. In the case of the MoCA and the drug trial, using a cutoff score of ≤22 (sensitivity = 94%) will provide good sensitivity (i.e., >90%, or failing to identify fewer than 1 in 10 patients judged to be incapable) to detect those patients deemed not capable of providing their own consent. In the case of the surgery trial, the same cutoff point for the MoCA of ≤22 provided sensitivity of 91%.
Table 3.
Sensitivity and specificity of the MMSE and MoCA
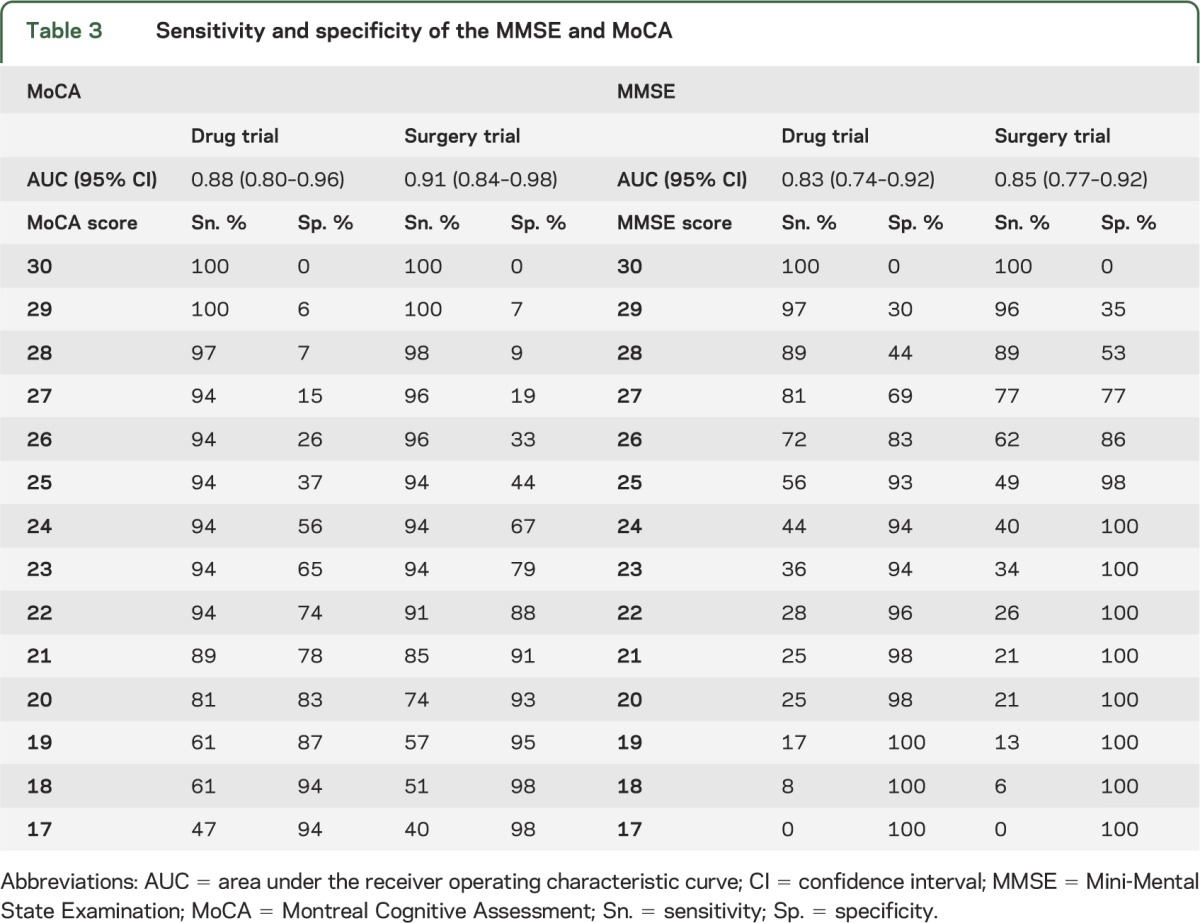
For the MMSE, the lowest adequate cutoff scores were ≤28, for both the drug and surgery trials, which is close to the ceiling of the instrument (i.e., score of 30). However, at this cutoff point, the specificity of the MMSE is far lower than the specificity of the MoCA at its comparable cutoff point, meaning that there will be a much higher percentage of false-positive results with the MMSE compared with the MoCA.
DISCUSSION
Among the requirements for an informed consent, an adult must have adequate capacity, but adults with PD may have cognitive impairments that cause diminished capacity.
Although cognitively normal PD patients generally were capable of consent, 17% were judged incapable of providing their own informed consent for the surgery trial. This is a proportion that, although comparatively smaller than the proportions of borderline and impaired PD patients judged to be not capable (43% and 97%, respectively), is arguably large enough that researchers enrolling such patients in high-risk trials ought to attend to decisional capacity. This result likely reflects that the more risky the clinical trial, the more likely experts are to judge a person not capable of consent.18
PD patients with borderline cognitive impairment are a clearly vulnerable group. Nearly half were judged not capable of providing their own informed consent to the surgery trial and one-third to the drug trial. This suggests that their typical impairments in at least one of the cognitive domains of memory, attention, and visuospatial and executive function may be clinically significant.29 These findings reiterate studies of treatment consent capacity in PD patients with MCI who also show impairments, particularly the ability to appreciate.3–5
In contrast to the medical decision-making studies, however, we found that most of the capacity impairments in PD patients with borderline or impaired cognitive function were related to the inability to understand information. This ability requires a person to encode facts, retain them, and map them onto his or her existing knowledge base. In our study, where subjects were allowed to retain the multipage consent form as they answered the understanding questions, they could search the form to find answers they could not recall. Impairments in understanding then reflect problems not simply in memorizing and recalling facts but in retrieving them from a multipage form, suggesting problems related to initiation and execution of an activity, problems common in the cognitive dysfunction seen in patients with PD.30 In a subsequent study, we will examine the relationships between DRS-2 subscales and measures of decisional ability.
PD patients with borderline cognitive impairment represent 25% to 30% of nondemented PD patients.31 Researchers who recruit them need to consider adopting subject protections over and above those they would take for cognitively normal PD patients. These might include a structured assessment of capacity, particularly the ability to understand information, and asking the patient to designate a study partner. Persons with Alzheimer disease retain the ability to designate a study partner even when they have lost the ability to provide their own informed consent to a study.17 Assuming that this same pattern is seen in patients with PD, then investigators can use this protection.
Categorizing patients into groups based on their cutoff scores on a measure of cognition sacrifices information, as categories miss information provided along this continuous dimension. Hence, we examined the odds of incapacity along the continuum of cognition. Both the MMSE and MoCA showed good ability to classify patients with PD as capable of consent to each of the 2 clinical trials. Perhaps the greatest value of these results is showing the range of MoCA scores that minimizes the error of mistakenly judging a patient as capable of consent who is in fact not capable. Our results suggest that this is a score of no less than 22.
Measuring cognition with the MoCA is not a substitute for a capacity assessment. Instead, the score helps researchers and clinicians better understand the kinds of patients who might have problems making a decision to enroll in more than minimal risk research. The sensitivity cutoffs may be useful to minimize the error of labeling a noncompetent patient as competent.
Our results only approximate a real clinical trial. In addition, we allowed subjects to retain the informed consent form during the capacity assessment. Other capacity assessment methods, such as giving persons a card with the relevant disclosure and then taking it away before assessing understanding, could yield different proportions of persons capable of consent. These limitations noted, we report valuable within-study comparisons of cognitively impaired PD patients with both normal controls and cognitively normal PD patients, and the criterion measure of expert judgments blinded to cognitive and capacity data.
PD patients with dementia typically have notable capacity impairments and those with MCI also have capacity deficits. In the case of more than minimal risk research, both groups should be considered a vulnerable population, and MoCA scores may be useful to identify patients at risk of capacity impairments.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients and normal controls who participated in this study, and Paige Brookstein, Abigail Darin, Eugenia Mamikonyan, James Minger, Jacqui Rick, and Baochan Tran for their assistance in subject recruitment and data gathering.
GLOSSARY
- AUC
area under the receiver operating characteristic curve
- CI
confidence interval
- DRS-2
Dementia Rating Scale–2
- MacCAT-CR
MacArthur Competence Assessment Tool for Clinical Research
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- PD
Parkinson disease
- ROC
receiver operating characteristic
Footnotes
Editorial, page 780
AUTHOR CONTRIBUTIONS
Jason Karlawish: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision, obtaining funding. Mark Cary: analysis or interpretation of data, statistical analysis. Stephen Moelter: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis, study supervision. Andrew Siderowf: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, study supervision. Elizabeth Sullo: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision. Sharon Xie: study concept or design, analysis or interpretation of data, statistical analysis. Daniel Weintraub: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision.
STUDY FUNDING
Research supported by National Institute of Neurological Disorders and Stroke R01NS65087 and Morris K. Udall Center for Parkinson's Disease Research core grants P50 NS053488 and NS062684.
DISCLOSURE
J. Karlawish has stock in SeniorBridge Inc., and is a coholder of a license of an Integrated NeuroDegenerative Disease Database developed at the University of Pennsylvania. M. Cary reports no disclosures. S. Moelter is a neuropsychological consultant for Avid Radiopharmaceuticals and is funded by NIH grants. A. Siderowf is a full-time employee of Avid Radiopharmaceuticals. E. Sullo reports no disclosures. S. Xie is a consultant for a clinical trial at Roche. D. Weintraub receives grant support from the NIH, Michael J. Fox Foundation for Parkinson's Research, Novartis Pharmaceuticals, and Department of Veterans Affairs. He receives personal compensation from Teva Pharmaceuticals, Lundbeck Inc., Biogen, Pfizer, Avanir Pharmaceuticals, Merck & Co., UCB, CHDI Foundation, and the Alzheimer's Disease Cooperative Study. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Weintraub D, Burn DJ. Parkinson's disease: the quintessential neuropsychiatric disorder. Mov Disord 2011;26:1022–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenthal E, Brennan L, Xie S, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord 2010;25:1170–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dymek MP, Atchison P, Harrell L, Marson DC. Competency to consent to medical treatment in cognitively impaired patients with Parkinson's disease. Neurology 2001;56:17–24 [DOI] [PubMed] [Google Scholar]
- 4.Griffith HR, Dymek MP, Atchison P, Harrell L, Marson DC. Medical decision-making in neurodegenerative disease: mild AD and PD with cognitive impairment. Neurology 2005;65:483–485 [DOI] [PubMed] [Google Scholar]
- 5.Martin RC, Okonkwo OC, Hill J, et al. Medical decision-making capacity in cognitively impaired Parkinson's disease patients without dementia. Mov Disord 2008;23:1867–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elgh E, Domellof M, Linder J, Edstrom M, Stenlund H, Forsgren L. Cognitive function in early Parkinson's disease: a population-based study. Eur J Neurol 2009;16:1278–1284 [DOI] [PubMed] [Google Scholar]
- 7.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangelos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308 [DOI] [PubMed] [Google Scholar]
- 9.Rubright J, Sankar P, Casarett DJ, Gur R, Xie SX, Karlawish J. A memory and organizational aid improves Alzheimer disease research consent capacity: results of a randomized, controlled trial. Am J Geriatr Psychiatry 2010;18:1124–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlawish JH, Casarett DJ, James BD. Alzheimer's disease patients' and caregivers' capacity, competency, and reasons to enroll in an early-phase Alzheimer's disease clinical trial. J Am Geriatr Soc 2002;50:2019–2024 [DOI] [PubMed] [Google Scholar]
- 11.Department of Health and Human Services Common Rule, 45 CFR 46. Federal policy for the protection of human subjects; notices and rules. Fed Regist 1991;56:28003–28032 [PubMed] [Google Scholar]
- 12.Appelbaum PS, Grisso T. The MacArthur Competence Assessment Tool—Clinical Research. Sarasota, FL: Professional Resource Press; 2000 [Google Scholar]
- 13.Appelbaum PS, Grisso T, Frank E, O'Donnell S, Kupfer DJ. Competence of depressed patients for consent to research. Am J Psychiatry 1999;156:1380–1384 [DOI] [PubMed] [Google Scholar]
- 14.Carpenter WT, Jr, Gold JM, Lahti AC, et al. Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry 2000;57:533–538 [DOI] [PubMed] [Google Scholar]
- 15.Dunn LB, Palmer BW, Appelbaum PS, Saks ER, Aarons GA, Jeste DV. Prevalence and correlates of adequate performance on a measure of abilities related to decisional capacity: differences among three standards for the MacCAT-CR in patients with schizophrenia. Schizophr Res 2007;89:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SYH, Caine ED, Currier GW, Leibovici A, Ryan JM. Assessing the competence of persons with Alzheimer's disease in providing informed consent for participation in research. Am J Psychiatry 2001;158:710–717 [DOI] [PubMed] [Google Scholar]
- 17.Kim SYH, Karlawish J, Kim HM, Wall IF, Bozoki A, Appelbaum PS. Preservation of the capacity to appoint a proxy decision maker: implications for dementia research. Arch Gen Psychiatry 2011;68:214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SY, Caine ED, Swan JG, Appelbaum PS. Do clinicians follow a risk-sensitive model of capacity-determination? An experimental video survey. Psychosomatics 2006;47:325–329 [DOI] [PubMed] [Google Scholar]
- 19.Karlawish JH, Kim SY, Knopman D, Van Dyck CH, James B, Marson D. Interpreting the clinical significance of capacity scores for informed consent in Alzheimer disease clinical trials. Am J Geriatr Psychiatry 2008;16:568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naglie G, Silberfeld M, O'Rourke K, et al. A randomized trial of a decisional aid for mental capacity assessments. J Clin Epidemiol 1993;46:221–230 [DOI] [PubMed] [Google Scholar]
- 21.Etchells E, Darzins P, Silberfeld M, et al. Assessment of patient competency to consent to treatment. J Gen Intern Med 1999;14:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlawish JH, Casarett DJ, James BD, Xie SX, Kim SY. The ability of persons with Alzheimer's disease (AD) to make a decision about taking an AD treatment. Neurology 2005;64:1514–1519 [DOI] [PubMed] [Google Scholar]
- 23.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442 [DOI] [PubMed] [Google Scholar]
- 24.Herzog J, Volkmann J, Krack P, et al. Two-year follow-up of subthalamic deep brain stimulation in Parkinson's disease. Mov Disord 2003;18:1332–1337 [DOI] [PubMed] [Google Scholar]
- 25.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1983;17:37–49 [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 27.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MOCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699 [DOI] [PubMed] [Google Scholar]
- 28.Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale–2. Lutz, FL: Psychological Assessment Resources; 2001 [Google Scholar]
- 29.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 2010;75:1062–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudlicka A, Clare L, Hindle JV. Executive functions in Parkinson's disease: systematic review and meta-analysis. Mov Disord 2011;26:2305–2315 [DOI] [PubMed] [Google Scholar]
- 31.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 2005;65:1239–1245 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


