Abstract
Background:
Current treatment strategies of psoriasis are not completely satisfactorily. By inhibiting inflammatory cytokines, nicotinamide may enhance the effects of current topical treatments. We investigated whether the combination of topical calcipotriol and nicotinamide is more effective than calcipotriol alone in treatment of psoriasis.
Materials and Methods:
Adult patients with mild to moderate psoriasis were randomized to receive topical calcipotriol 0.005% and nicotinamide 4% in combination or calcipotriol 0.005% alone, twice daily for 12 weeks. Patients were visited by a dermatologist at baseline and then after the first and third month of therapy, and psoriasis severity was evaluated using the modified psoriasis area and severity index (PASI). Also, patient's satisfaction was evaluated at the end of the trial using a 10-point rating scale.
Results:
Sixty-five patients (35 males, mean age = 36.5 ± 8.5 years) completed the trial. Lesions on both sides were similar regarding baseline PASI score. At the end of the trial, PASI score was more reduced with calcipotriol+nicotinamide compared to calcipotriol alone (83.6 ± 7.9% vs. 77.8 ± 9.7%, P < 0.001). Patients were also more satisfied with the improvement of lesions with calcipotriol+nicotinamide compared with calcipotriol alone (P < 0.001). Side effects included mild erythema and pruritus (4.6%) and moderate burning and sensitivity to light (3.0%).
Conclusions:
Nicotinamide can enhance the efficacy of calcipotriol when used in combination for topical psoriasis treatment, and it may be a good adjuvant to the current treatment regimens of psoriasis.
Keywords: Calcipotriene, calcipotriol, nicotinamide, psoriasis, therapy
INTRODUCTION
Psoriasis is a chronic skin disorder typically characterized by symmetrical erythematous papules and plaques with a silver scale. Psoriatic plaques involve the scalp, extensor elbows, knees, and the back. Population-based studies showed a worldwide prevalence of 0.6% to 4.8% for psoriasis.[1] Although current treatment strategies are not sufficiently effective for this disease, there are several treatments regimens that are suggested (as monotherapy or in combination with other drugs) to control psoriasis. Mild to moderate disease is treated with topical medications, and, for more severe disease, adding systemic therapies to topical regimes are recommended. The most common prescribed topical treatments for psoriasis are corticosteroids, which reduces the skin inflammation and irritability.[2]
Calcipotriol is one of the vitamin D analogs that has been used for psoriasis as topical monotherapy or in combination with other medications; however, its use is limited because of its moderate efficacy.[2,3] Nicotinamide is a vitamin B derivative that, according to preliminary studies, reduces the proliferation of keratinocytes in the human skin.[4] Thus, the adjuvant therapy of nicotinamide and calcipotriol may increase the therapeutic effects as compared with monotherapy. In a pilot study by Levine et al., on patients with moderate psoriasis, the effect of calcipotriol alone or with various doses of nicotinamide was evaluated. This study found that the combination regime of 0.005% calcipotriene and 1.4% nicotinamide was effective in the treatment of psoriasis;[5] however, the study sample size limited the results to show statistically significant differences. Hence, the goal of the current study was to investigate the beneficial effects of adding nicotinamide to calcipotriol for patients with mild to moderate psoriasis.
MATERIALS AND METHODS
Patients and settings
This randomized, double-blinded, controlled trial was conducted on patients with psoriasis referring to the outpatient clinics of dermatology in three university clinics (Alzahra and Noor Hospitals and Sedighe Tahere Research Center) in Isfahan (Iran) between March 2011 and June 2012. Patients included into our study were men and women of age 18-65 years, with mild to moderate psoriasis; patients must have had <15% of the involved body surface, symmetrical plaques (bilateral lesions) or two plaques at least 5-cm apart on the same side of the body with plaque size greater than 2 × 2 cm, but smaller than 15 × 15 cm. Patients with the following characteristics were not included into the study; those who used any medication or niacin and multi-vitamins for 2 weeks or anti-psoriatic systemic drugs or beta-blockers 1-month prior to the study, pregnant women, those with the history of renal, hematologic, liver and major psychiatric diseases, and those with only scalp, nail, flexural, palmoplantar, or pustular psoriasis. With regard to type I error (alpha) = 0.05, study power = 80%, and expected difference of 30% in response rate, sample was calculated as 65 patients in each group. The study was approved by the ethics committee of the Isfahan University of Medical Sciences, and all patients signed a written inform consent before entering the trial. Also, the study is registered at ClinicalTrials.gov (NCT01763424).
Intervention
Patients who fulfilled the inclusion criteria were consecutively entered into the study. The plaques in each individual case that were two lesions at two opposite side (right and left) were coded (codes 1 and 2). We used random allocation software[6] to select the codes to either receive calcipotriol 0.005% and nicotinamide 4% in combination or calcipotriol 0.005% alone (LEO Pharmaceuticals, Ballerup, Denmark). Nicotinamide 4% was used according to preliminary data on its dose-response effect for psoriasis,[5] and safety of this dose was used for acne treatment.[7,8] The patients used the medications twice daily (in the morning and before sleeping) for 12 weeks; the total doses of medication used was not >100 g/week.
Assessments
Patients were visited by a dermatologist at baseline and after the first and third month of therapy. In each visit, psoriasis severity was evaluated using the modified psoriasis area and severity index (PASI) for lesions of each side. The PASI is commonly considered as a denominator for satisfactory results of any treatment modality for psoriasis. With PASI, the severity of psoriasis is measured based on the severity of four affected sites; the head (h), upper limb (u), trunk (t), and lower limbs (l). Each site is separately scored by using three parameters of erythema (E), induration (I), and desquamation (D).[9] We used modified PASI because we evaluated the body lesions.[10] The score ranges from 0 to 60, representing the proportion of the involved area and the severity of erythema, infiltration, and desquamation, ranking from 0 (normal) to 4 (severe). The affected area is registered and, according to the expansion, valued in score points from 0 to 5 points. The proportion of area involved was as follows: Area (%): 0 = 0; 1 to 20 = 1; 21 to 40 = 2; 41 to 60 = 3; 61 to 80 = 4; and 81 to 100 = 5 score points. Thus, the modified PASI score = affected surface area in multiply with the sum of erythema and infiltration and desquamation. Thereafter, PASI score of one side of the lesions were wholly compared to that in the other side. To evaluate the patient's satisfaction from each side of treatment, a 10-point self-rated numerical rating scale was used at the end of the trial.
Statistical analysis
The data were analyzed using the SPSS software (version 16.0) for windows. Quantitative variables are presented as mean ± SD and modified PASI score is presented based on the percentage of changes. Independent sample t-test was used for comparing the qualitative variables and paired t-test was used for comparing the quantitative variables before and after the intervention in each group. P < 0.05 was considered significant in all analyses.
RESULTS
During the study period, we evaluated 77 patients, from which 7 patients did not match the inclusion criteria and 4 were not willing to participate. Also, during the study period, one patient discontinued the trial due to dissatisfaction with therapy. Thus, a total number of 65 patients (35 males) with mean age of 36.5 ± 8.5 (22-56) years completed the trial. The PASI score of each side of the cases at three measurements are presented in Table 1. Analyses showed that lesions on both sides were similar regarding baseline PASI score (P = 0.148), while at 1 month and 3 months after therapy, PASI scores were significantly lower with calcipotriol+nicotinamide as compared with calcipotriol alone (P < 0.001). At the end of the trial, PASI scores were reduced by 22.4 ± 8.8 points with calcipotriol+nicotinamide as compared to 20.3 ± 7.6 points with calcipotriol alone (83.6 ± 7.9% vs. 77.8 ± 9.7% reduction, P < 0.001). The trend of changes in PASI score of each side lesions are presented in Figure 1.
Table 1.
Comparison of PASI scores between lesions on A with that on B
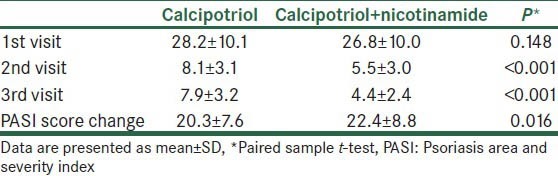
Figure 1.
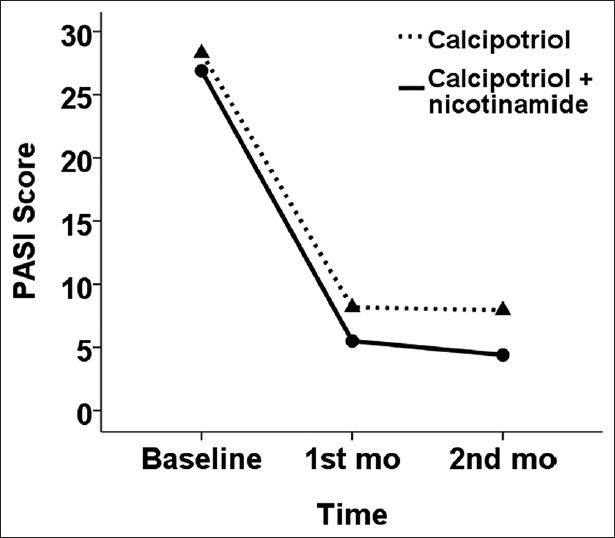
Trend of changes in PASI scores with two therapies
Patients were more satisfied regarding improvement of lesions for which they had used calcipotriol+nicotinamide as compared with calcipotriol alone (6.5 ± 1.4 vs. 5.5 ± 1.6, P < 0.001). Side effects included mild erythema and pruritus (4.6%) and moderate burning and sensitivity to light (3.0%). No patients discontinued the study as a result of adverse events.
DISCUSSION
Current therapies of psoriasis are not completely satisfactorily. Topical steroids are the most frequently used treatments for psoriasis; however, because of various unwanted effects of long-term use of corticosteroids, such as infections, drug dependency, and skin breakdown, asserting to improve more corticosteroid-sparing regimens has been under attention for the treatment of psoriasis. These agents include vitamin D-analogs (e.g., calcipotriol and tacalcitol), vitamin A-analog (e.g., tazarotene), tars, and topical immunosuppressants as single agents or in combination with other treatments.[11] However, drug agencies warning indicates that calcineurin inhibitors and immunosuppressants can cause malignancy in these patients; therefore, their long-term use in all patients is under question and is suggested to be limited.[12] Recent studies showed that calcipotriol is safe for long-term use in psoriasis. It can be used in combination with corticosteroids or alone. Studies showed a marked reduction of psoriatic plaques in the treatment with calcipotriol and corticosteroids including betamethasone. Also, these combinations were well tolerated by patients.[11,13,14,15]
Preliminary studies have shown that nicotinamide, which is a vitamin B derivative, is effective in the treatment of psoriasis.[5] Because of the lack of data, we investigated the beneficial effects of adding nicotinamide to calcipotriol for patients with mild to moderate psoriasis in a relatively large sample of patients. Our data showed that calcipotriol with combination of nicotinamide is more effective in reducing the patient's symptoms and also regarding treatment satisfaction in psoriasis while exerting no specific or severe adverse effects. In another double-blinded trial, Levine et al., divided patients with psoriasis into groups of calcipotriene 0.005% alone, nicotinamide 1.4% alone, and calcipotriene 0.005% plus nicotinamide 0.05%, 0.1%, 0.7%, and 1.4%. Trial was continued for 12 weeks and authors found that half of the patients in nicotinamide+calcipotriene group achieved a good response to the treatment as compared with 18.8% with placebo, 25% with nicotinamide alone, and 31.5% with calcipotriene alone. Authors concluded that 0.005% calcipotriene+1.4% nicotinamide is the best concentration of this combination.[5] This study also showed a dose-response effect for nicotinamide. Accordingly, we selected a higher dose that has been shown to be safe in acne treatment[7,8] and found that the dose was highly effective in treatment of psoriasis, while it did not have specific or severe adverse effects. Besides these two double-blinded trials on nicotinamide, Zackheim compared 6-aminonicotinamide (6-AN, a nicotinamide analog) with different topical steroids (e.g., triamcinolone) and reported substantial improvement with 6-AN compared with steroids. Investigators also reported mild and reversible adverse effects as tachyphylaxis, mucocutaneous toxicity, and tinnitus.[16] Overall, these results introduce nicotinamide as a useful adjuvant to the treatment regimens of psoriasis.
The exact mechanisms by which vitamin A and D analogs can affect the psoriatic plaque are not clear; however, they may reverse the keratinocytes hyperproliferation by imonohistochemical pathways. Psoriasis is an inflammatory disorder characterized with the increased expression of T-helper cell 1 and T-helper cell 17 cytokines. In addition, the expression of adhesion molecules, neutrophil accumulation, and production of nitric oxide are increased in psoriasis. Moreover, histamine and proteases are claimed in this pathogenesis. Nicotinamide combinations prevent the expression of inflammatory cytokines, chemokines, adhesion molecules, and inflammatory mediators by inhibiting the nuclear kappa B-mediated transcription. Furthermore, it is a phosphodiesterase inhibitor that can suppress the neutrophil and mast cells activity; in this manner, nicotinamide inhibits nitric oxide syntheses by lymphocyte, hence these mechanisms suggest nicotinamide as an anti-skin inflammatory and anti-psoriatic agent.[4]
There are some limitations for our study. The modified PASI score that we used for the assessment of psoriasis has the limitation of inter-observer variations. Also, the duration of 12 weeks of therapy was not sufficient to demonstrate the complete effects and side effects of using nicotinamide as a long-term treatment for psoriasis.
CONCLUSIONS
Nicotinamide can enhance the efficacy of calcipotriol when used in combination for topical psoriasis treatment and may be a good adjuvant to be added to the treatment regimens of psoriasis. Further trials with long-term follow-up are required to confirm these results and also to evaluate possible adverse effects with long-term use of nicotinamide.
ACKNOWLEDGMENTS
This study was supported as a thesis of Specialty in Dermatology by the Isfahan University of Medical Sciences (Grant number 390134). Authors are thankful to Dr. Leila Mirbagher for statistical advices and for editing this report.
Footnotes
Source of Support: Isfahan University of Medical Sciences
Conflict of Interest: None declared
REFERENCES
- 1.Naldi L. Epidemiology of psoriasis. Curr Drug Targets Inflamm Allergy. 2004;3:121–8. doi: 10.2174/1568010043343958. [DOI] [PubMed] [Google Scholar]
- 2.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643–59. doi: 10.1016/j.jaad.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 3.Mason AR, Mason J, Cork M, Dooley G, Edwards G. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2009;15:CD005028. doi: 10.1002/14651858.CD005028.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Namazi MR. Nicotinamide: A potential addition to the anti-psoriatic weaponry. FASEB J. 2003;17:1377–9. doi: 10.1096/fj.03-0002hyp. [DOI] [PubMed] [Google Scholar]
- 5.Levine D, Even-Chen Z, Lipets I, Pritulo OA, Svyatenko TV, Andrashko Y, et al. Pilot, multicenter, double-blind, randomized placebo-controlled bilateral comparative study of a combination of calcipotriene and nicotinamide for the treatment of psoriasis. J Am Acad Dermatol. 2010;63:775–81. doi: 10.1016/j.jaad.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morganti P, Berardesca E, Guarneri B, Guarneri F, Fabrizi G, Palombo P, et al. Topical clindamycin 1% vs. linoleic acid-rich phosphatidylcholine and nicotinamide 4% in the treatment of acne: A multicentre-randomized trial. Int J Cosmet Sci. 2011;33:467–76. doi: 10.1111/j.1468-2494.2011.00658.x. [DOI] [PubMed] [Google Scholar]
- 8.Dos SK, Barbhuiya JN, Jana S, Dey SK. Comparative evaluation of clindamycin phosphate 1% and clindamycin phosphate 1% with nicotinamide gel 4% in the treatment of acne vulgaris. Indian J Dermatol Venereol Leprol. 2003;69:8–9. [PubMed] [Google Scholar]
- 9.Fredriksson T, Pettersson U. Severe psoriasis: Oral therapy with a new retinoid. Dermatologica. 1978;157:238–44. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 10.Feldman SR. A quantitative definition of severe psoriasis for use in clinical trials. J Dermatolog Treat. 2004;15:27–9. doi: 10.1080/09546630310019382. [DOI] [PubMed] [Google Scholar]
- 11.Bailey EE, Ference EH, Alikhan A, Hession MT, Armstrong AW. Combination treatments for psoriasis: A systematic review and meta-analysis. Arch Dermatol. 2012;148:511–22. doi: 10.1001/archdermatol.2011.1916. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann R, Bibby AJ, Bissonnette R, Cambazard F, Chu AC, Decroix J, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389–93. doi: 10.1159/000066440. [DOI] [PubMed] [Google Scholar]
- 13.Duweb G, Alhaddar J, Elsherif B, Eljehawi N, Makhlouf H. Calcipotriol-betamethasone ointment versus calcipotriol ointment in the treatment of psoriasis vulgaris. Drugs Exp Clin Res. 2005;31:175–9. [PubMed] [Google Scholar]
- 14.Kragballe K, Hoffmann V, Ortonne JP, Tan J, Nordin P, Segaert S. Efficacy and safety of calcipotriol plus betamethasone dipropionate scalp formulation compared with calcipotriol scalp solution in the treatment of scalp psoriasis: A randomized controlled trial. Br J Dermatol. 2009;161:159–66. doi: 10.1111/j.1365-2133.2009.09116.x. [DOI] [PubMed] [Google Scholar]
- 15.Luger TA, Cambazard F, Larsen FG, Bourcier M, Gupta G, Clonier F, et al. A study of the safety and efficacy of calcipotriol and betamethasone dipropionate scalp formulation in the long-term management of scalp psoriasis. Dermatology. 2008;217:321–8. doi: 10.1159/000155642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zackheim HS. Topical 6-aminonicotinamide plus oral niacinamide therapy for psoriasis. Arch Dermatol. 1978;114:1632–8. [PubMed] [Google Scholar]


