Summary
The term “adrenal incidentaloma” refers to clinically unapparent adrenal mass detected during imaging examination performed for reasons other than the evaluation of adrenal glands. These tumors must be carefully examined in order to assess the indications for surgical treatment. The main method of finding evidence of potential malignancy in these lesions is computed tomography (CT), before and after i.v. contrast media enhancement. Density of a malignant lesion is higher than 10 HU and the relative percentage washout is less than 40% at 10 min. Other useful methods utilized in tumor assessment, include magnetic resonance imaging (MRI), scintigraphy techniques (SPECT) and PET. Basal hormonal investigations include urine and plasma catecholamines with their metabolites, plasma cortisol before and after dexamethasone administration, plasma renin activity and aldosterone level. Cases not suitable for surgery should be followed with repeat imaging techniques and hormonal testing at the recommended 6, 12, and 24 months. Surgery should be performed when tumor growth rate exceeds 0,8 cm per year.
Keywords: adrenal incidentaloma, adrenal adenoma, adrenal cancer, pheochromocytoma, pre-Cushing’s syndrome
Background
The term ‘incidentaloma’ refers to adrenal gland tumors greater than 1 cm in diameter and detected through an imaging procedure performed for unrelated reasons. Incidentally discovered adrenal masses are becoming more common with the development of imaging techniques and constitute a significant clinical problem. Adrenal tumors are detected in approximately 0.4% of abdominal ultrasounds (US) performed for various reasons, 10 times more commonly in patients with a history of neoplastic disease. According to some reports, adrenal tumors are accidentally discovered in 4–5% of patients [1,2] undergoing abdominal CTs performed for non-endocrinological reasons; however, some authors cite a lower incidence – up to 1% [3]. Very small adrenal masses, <1 cm in diameter, were found in as much as 65% of postmortem adrenal gland examinations. These masses are associated with changes taking place in the organ in the course of a physiological process of aging [4,5]. Solitary lesions greater than 1 cm in diameter were found in 8.7% of autopsies performed in persons older than 65 years [6]. More recent studies report a mean occurrence of 6% in the general population, whereas the incidence determined on autopsy and through diagnostic imaging varies between 1–32% and 0.2–7%, respectively [7,8]. Incidence of adrenal lesions before the 30th year of life does not exceed 1%, whereas after the age of 70 it is greater than 7% [9,10].
Focal lesions commonly develop in the adrenal glands. However, surgery is only indicated in a small fraction of such cases, because mild and hormonally inactive lesions predominate among them. Differential diagnosis workup carried out after an incidental detection of adrenal tumor must provide answer as to whether surgery or observation is indicated. Such diagnostic procedure requires close co-operation between radiologist and endocrinologist. Indications for surgical treatment of adrenal incidentalomas can be based on incidentaloma’s potential for malignancy determined by imaging or hormonal activity that is assessed with a few simple tests.
Diagnostic Imaging
Ultrasonography (US)
Adrenal tumors are most commonly found during abdominal ultrasound (US). It is not always possible to visualize normal adrenal glands (especially on the left side) with an ultrasound [11,12]. Their physiological thickness does not exceed 12 mm, while the sensitivity of this method is over 90% for diagnosing tumours 2 cm in size [13,14]. Apart from lesion measurement, ultrasonography enables assessment of calcifications, foci of necrosis or cysts. However, this method provides no answer to the question, whether the tumor is malignant or benign. Tumor malignancy potential can only be estimated on the grounds of its dimensions: about 2% of carcinomas are found incidentally in adrenal tumors less than 4 cm in diameter and 6% in tumors 4–6 cm in diameter. About 90% of adrenal carcinomas have a diameter of over 4 cm, but only every fourth tumor larger than 6 cm in diameter is an adrenal carcinoma [15–17].
Computed tomography (CT)
Computed tomography performed before and after intravenous (i.v.) administration of contrast medium is the most useful imaging technique for adrenal incidentaloma evaluation.
Qualitative tumor evaluation
Qualitative parameters are tumor shape and homogeneity, which often preliminarily suggest its character. Benign adenomas are usually oval, homogeneous and well-delimited lesions (Figure 1) [18]. Tumor with an irregular shape, areas of necrosis, posthemorrhagic foci, calcifications, non-homogeneity, is rather a malignant change (Figure 2) [19]. However, benign pheochromocytomas can also be inhomogenous and contain calcifications or foci of necrosis, whereas the shape is usually more regular than that of malignant changes (Figure 3) [20]. A mild adenoma with a posthaemorrhagic focus can be also inhomogeneous [21]. Mild myelolipomas can be very inhomogeneous [22,23].
Figure 1.
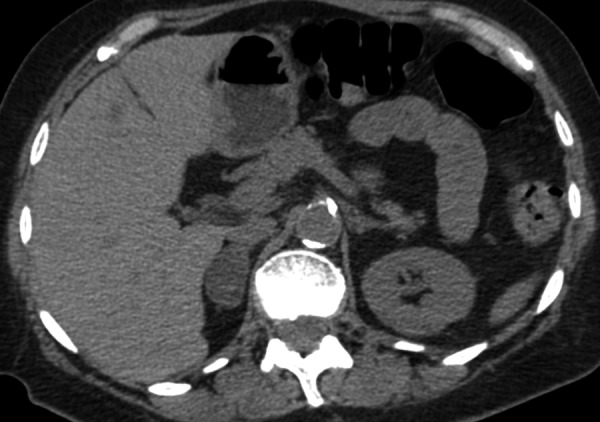
Axial CT scan without contrast enhancement, showing right adrenal adenoma, which typically exhibits low density that is less than that found in the liver, spleen and kidneys due to high fat content.
Figure 2.
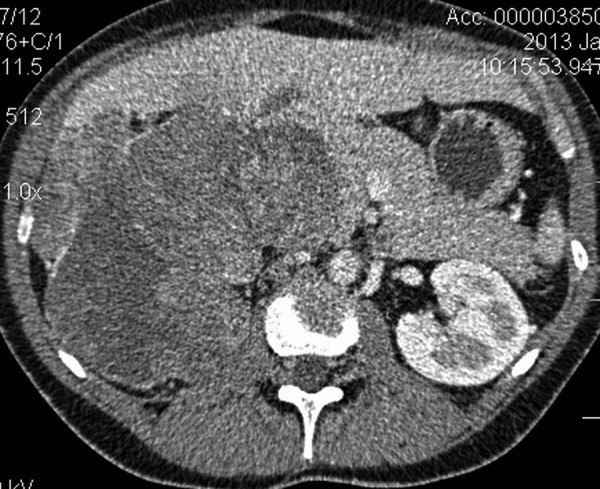
Axial CT scan after i.v. contrast enhancement – arterial phase (30 s), showing large mass compressing the right lobe of the liver, right retroperitoneal space involvement and compression of IVC. Image displays malignant adrenal tumor, histologically confirmed as adrenocortical carcinoma.
Figure 3.
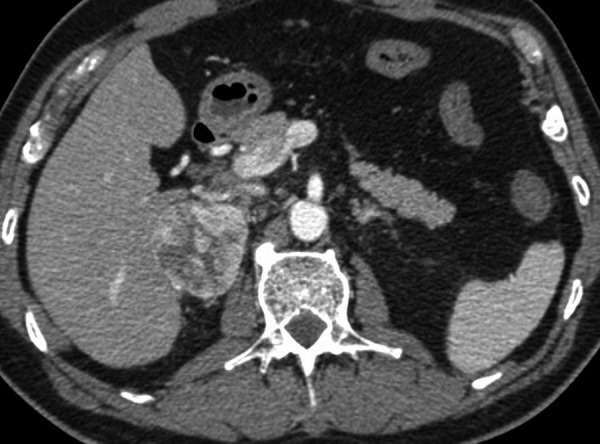
Axial CT scan after i.v. contrast enhancement – arterial phase (30 s), showing heterogeneous high contrast enhancement in the right adrenal mass that could be suspicious for pheochromocytoma.
Tumor size
Tumour size is the first quantitative parameter assessed. In case of cross-sectional images made every 3–5 mm, lesions greater than 5 mm in diameter can be visualized [19]. Most of the tumors ≤3 cm in diameter are mild lesions, whereas most of the tumors greater than 6 cm in diameter are malignant lesions. Since all malignant tumors had a diameter <3 cm at the beginning of their „natural history”, the above rule may not apply. On the other hand, mild adenomas without secretory activity may grow for a very long time and become larger than 6 cm in diameter. Mild myelolipomas commonly grow above 6 cm [10,21]. Thus, tumor size is only a supplementary element in CT scan description and should not be used as the only basis for therapeutic decisions. Any attempts at formulating indications for surgery based solely on the tumor size will result in criteria that range from 4 cm to 6 cm depending on the country or medical center. Changes in tumor size during the observation time constitutes a separate end point. A rapid increase in tumor size (>0.8 cm/year), even in the „mild phenotype”, is an indication for tumor resection [24]. On the other hand, metastatic lesions not growing for 3 years were described. Furthermore, 5–25% of mild lesions grow significantly larger during 1-year observation according to various data [10].
Radiation attenuation coefficient
The evaluation of radiation attenuation coefficient, i.e. the so-called density, is the most important element of CT scan description of a tumor. Density is expressed in Hounsfield units (HU) and represents the value of X-radiation attenuation linear coefficient for the examined tissue in comparison with the coefficient measured for water. The range is 2000 HU: the coefficient for water is 0 HU, for air (–) 1000 HU, for adipose tissue about (–) 100 HU and for soft tissues (+) 20 – (+) 70 HU [25]. During diagnostic workup for adrenal tumors, density should be assessed before and 1 minute after the administration of i.v. contrast enhancement, followed by acquisition (washout phase) 10 or 15 minutes later. The so-called imaging phenotype of a tumor, which can be „mild” or „suspicious”, can be assessed on the grounds of measurements recorded in this protocol. A tumor with mild imaging phenotype is one with low density, i.e. ≤10 HU (Figure 4A, 4B). This low density means that the lipid fraction of the tumor is high, which is characteristic of lipid-rich mild adenoma. In principle, an initial density <10 HU may not require further density assessment following contrast agent administration. However, approximately 30% of adenomas are lipid-poor adenomas, i.e. mild ones, but they are characterized by high initial density (usually up to 20 HU and in some cases up to 30 HU). Density assessment following i.v. contrast administration is necessary in such cases. Moderate enhancement, followed by a rapid contrast agent washout from the tumor occurs in adenomas. Calculation of the so-called contrast washout coefficients expressed in% is based on differences in density. An absolute coefficient is a quotient in which the difference between maximal density (during the 1st minute) and the density after 10 or 15 minutes, is the numerator; while the difference between the maximal value and the initial value is the denominator (result is multiplied by 100%). This parameter is used for examinations performed according to the adrenal protocol with initially planned subsequent duration of exposure. Upon discovery of adrenal tumor during the vascular phase on thorax CT scan and no initial density value, the relative coefficient can be used. It represents the proportion between the decrease in density from the 1st minute until the 10th minute of examination and the density during the 1st minute. An absolute washout coefficient that is greater than 50% after 10 minutes, 60% after 15 minutes and a relative washout coefficient that is greater than 40% indicates a mild character of the lesion. This allows the diagnosis of a lipid-poor adenoma, i.e. a lesion that requires surgery only in case of confirmed hormonal activity (ACTH-independent hypercortisolemia or primary hyperaldosteronism) [10,15,19,21,24–26].
Figure 4.
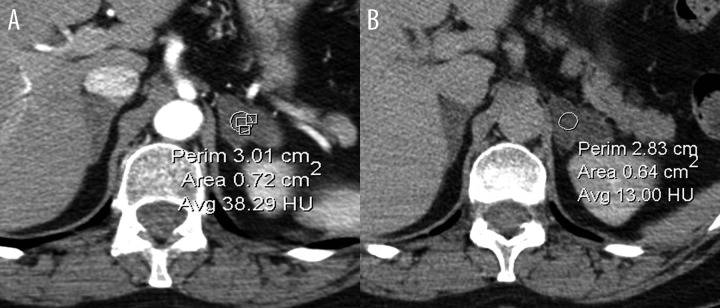
Axial CT scan of a left adrenal tumor after i.v. contrast enhancement. Initial scan done after 60 s (A) followed by 10 min acquisition (washout phase) (B). Typical appearance of adrenal adenoma exhibiting moderate enhancement, followed by rapid contrast agent washout from the adenoma.
Myelolipomas are heterogenous and display an extremely low density. They rarely occur in the adrenal glands and are often exceptionally large in size, therefore raising concern. Presence of fatty foci with an attenuation coefficient from less than – 30 HU to – 100 HU is characteristic of this mild tumor. Myelolipomas can also contain small calcifications [22,23].
Definitive need for surgery occurs in cases in which values characteristic of lipid-poor adenoma are not confirmed by a contrast washout coefficient determination for a tumor with initial high density. However, further differential diagnosis is essential for a correct preparation of patients for surgery (necessary administration of alpha and beta blockers prior to the operation of pheochromocytoma) and for the choice of operative technique (preferred laparoscopic approach for pheochromocytoma and open operative technique for malignant lesions). However, such management is not simple with CT scans (Figures 2 and 3). As mentioned above, primary adrenocortical carcinoma is inhomogenous and therefore it is possible to distinguish areas with a lower density (foci of necrosis) and areas with a very high density (calcifications). Immediately after contrast agent injection the density of a neoplastic tumor increases variously according to the degree of pathological vascularization. Washout is slower in comparison to adenomas. The differences in density of various tumor areas found during the initial phase, may sometimes decrease after contrast medium administration and the tumor may be taken as a homogenous one. An often centrally located focus of disintegration can also become visible [27,28]. Metastatic tumors have a similar phenotype with less frequent calcifications. Pheochromocytomas derived from the adrenal medulla may have a similar high density and calcifications (Figure 3). This kind of pheochromocytomas shows a stronger enhancement after intravenous injection of contrast agent and usually a slower washout than that of adenomas, but similar to that of carcinomas [20]. The imaging phenotype of pheochromocytoma can successfully imitate the phenotype of adrenocortical carcinoma. We therefore attempted to use other methods described below for its identification.
Magnetic resonance imaging (MRI)
Computed tomography is complemented by magnetic resonance imaging. MRI evaluation includes T1- and T2-weighted images, an assessment of lipid content by chemical shift of MRI (Figure 5A, 5B) and after i.v. contrast enhancement. Examination by chemical shift MRI consists of an assessment of T1-weighted GRE images in the so-called phase (in-phase) and antiphase (out-of-phase). MRI of adrenocortical adenoma is homogeneous, whereas the signal intensity is low – approximating the signal from the liver and also often lower than in the other parts of adrenocortical tissue. Except for cases in which adrenal adenoma had hemorrhage, which produces high signal intensity in T1 weighted out of phase images [29]. Signal intensity within adrenocortical carcinomas and pheochromocytomas is significantly higher and heterogeneous on T1- and especially on T2-weighted images, as well as on DWI (Figure 6A–6C). Since they contain lipids, mild lesions, i.e. adenomas, adrenocortical hyperplasia and myelolipomas, are characterized by a decreased signal intensity in the antiphase (out-of-phase). Trace amounts of lipids can also occur in adrenal carcinomas, whereas pheochromocytomas almost never contain lipids [19,29]. Thus, this technique may help distinguish carcinomas from pheochromocytomas, however, one has to bear in mind that mixed tumors with adrenal cortical and medullary texture, i.e. containing lipids, but also constituting a source of catecholamine excess, were also described. The differences in contrast enhancement washout between malignant tumors and adenomas, are similar to those seen on CT scans [30].
Figure 5.
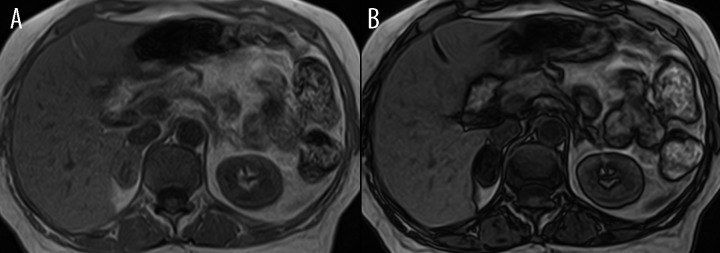
MRI scan performed in-phase (A) and out-of-phase (B) showing a right adrenal adenoma exhibiting the typical decrease in signal intensity on the out-of-phase image. Confirmed histologically as adrenocortical adenoma.
Figure 6.
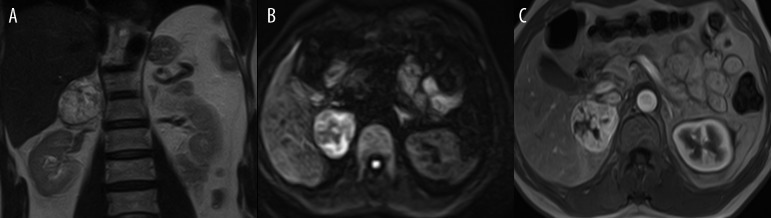
Coronal T2-weighted image (A), axial DWI (diffusion weighted image B=125 (B) and axial 3-dimensional GRE sequence with contrast-enhanced MR image and (C) showing a right adrenal pheochromocytoma. The pheochromocytoma shows the typical features of high signal intensity in T2 images and DWI, as well as intense enhancement following administration of contrast.
Functional Imaging – Radioisotope Imaging
Radioisotope examinations using scintigraphy or positron emission tomography (PET) should be considered in cases where tumor character was not clearly determined by a CT or an MRI, in clinical and/or biochemical suspicions of pheochromocytoma and in case of bilateral potentially secreting adenomas.
The cholesterol derivative, 131I-6-iodomethylnorcholesterol (NP-59), is a radiotracer that is selectively taken up in the adrenal cortex. It accumulates bilaterally in the normal adrenal glands and unilaterally in case of a hormonally active adenoma or carcinoma. Labelled 123I-meta-iodobenzylguanidine (mIBG) is taken up by pheochromocytoma cells (some polish centers use an outdated 131I mIBG) (Figure 7A–7C). Malignant pheochromocytomas or paragangliomas could be 123I mIBG avid tumors, but more often had over-expression of somatostatin receptors, that enable their visualization by SPECT scanning using 99mTc-[HYNIC,Tyr3]-octreotide (Tektrotyd) (Figure 8A–8C) or 68Ga DOTATATE PET [31,32].
Figure 7.
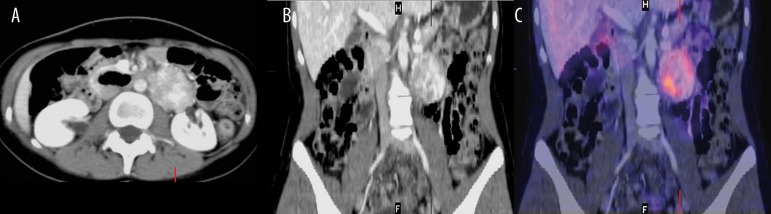
Axial CT scan after i.v. contrast enhancement (60 s) showing highly enhanced mass in the left adrenal (A), image fusion CT and 123I mIBG scintigraphy (SPECT technique) (B), that represents pheochromocytoma of the left adrenal gland.
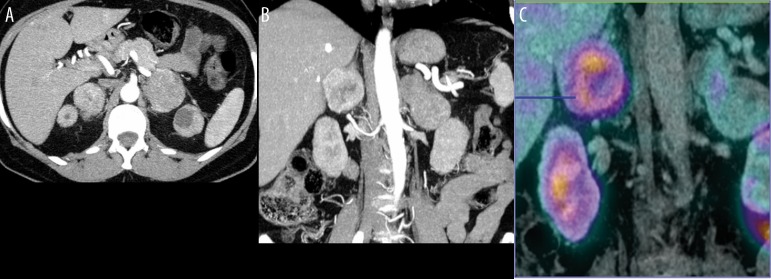
Figure 8.
Axial CT scan after i.v. contrast enhancement (60 s) showing two adrenal masses with high enhancement within two adrenal tumors (A), CT – coronal view (B), highly suspicious of MEN 2A pathology, previous history of medullary thyroid carcinoma – MTC). Additional examination with somatostatin receptor scintigraphy, 99Tc Tektrotyd (SRS), presented as image fusion with CT (C), showing high radiotracer uptake in the right pheochromocytoma, with weak, peachy uptake of the left pheochromocytoma. Metabolic profile of these confirmed bilateral pheochromocytomas was different.
11C-metomidate (an inhibitor of steroidogenesis) is taken up in adenomas, showing a homogenous distribution and is used in PET technique for specific imaging of steroid hormone-producing tumors. Metomidate is taken up with the same intensity in adrenal carcinomas, but its distribution in the tumor is not homogenous [33]. 11C-meta-hydroxyephedrine, an amine not undergoing enzymatic breakdown by MAO with a molecular structure approximating that of noradrenaline, is a radiotracer that allows very precise imaging of adrenal medullary tumors. However, it has a very short half-life, which limits its use. Radiolabelled 11C-adrenaline, or 18F-dopamine and dihydroxyphenylalanine are used in PET imaging of pheochromocytomas [19,34].
18F-fluorodeoxyglucose (FDG) is a nonspecific tracer that is applied for PET examination of different organs. 18FDG is usually not taken up in mild adenomas, but it visualizes metastases to the adrenal glands and also pheochromocytomas (particularly the rapidly growing ones). However, it should be emphasized that mild lesions with high metabolic and secretory activity can also display an intensive FDG uptake [19,28,35].
The main advantage of diagnostic imaging is the ability to precisely distinguish benign adenomas from malignant tumors, i.e. primary or metastatic tumors and pheochromocytomas that require prompt surgery. Tumor imaging is usually performed by computed tomography and less frequently by magnetic resonance imaging. In order to ensure good quality CT assessment of adrenal glands, administration of contrast enhancement with an injector is required. This allows for radiogram timing standardization in relation to contrast enhancement of lesion. Determination of lipid content on MRI requires an extension of the scope of routine examinations of abdominal organs.
Evaluation of Tumor Hormonal Function
Incidentalomas are detected in non-endocrinological diagnostics. According to the definition, clinical features of adrenal hormone excess should not be present. An incidentally discovered adrenal tumor in patients with poor diagnostics for paroxysmal arterial hypertension or with unidentified clear features of hypercortisolemia can not be recognized as an incidentaloma. However, there are cases with a subclinical hormonal function, which necessitate adrenal tumor resection, even when the tumor does not produce overt clinical symptoms and its imaging phenotype is unmistakably a mild one.
Subclinical Cushing’s Syndrome
Autonomous cortisol secretion occurs in approximately 5% of adrenal incidentaloma cases [8]. In fact, subclinical Cushing’s syndrome despite the suggestion contained in this name, is not completely asymptomatic. It was demonstrated that several disturbances can be detected in patients with „subtle” hormonal hyperfunction without visible somatic features of Cushing’s syndrome, as well as the evident effects of hypercortisolemia in the organs. These disturbances include: an increase in body mass index, waist-to-hip ratio, lipid concentrations, incidence of obesity and impaired glucose tolerance or even of type 2 diabetes [36]. Increased values of systolic and diastolic arterial pressure, insulin resistance, a significantly thicker vascular wall and a greater number of atheromatous lesions in the vessels are found in most patients with apparent clinically-silent adrenal adenoma [16,37]. These patients also have a lower bone mineral density [38]. In other words, an apparent clinically-silent adrenal adenoma can not cause metabolic syndrome, arterial hypertension, osteoporosis, nor increase the risk of diabetes [39]. Thus, it may be assumed that a subclinical hormonal hyperactivity of a tumor may constitute an indication for surgery [40].
Patients with subclinical hypercortisolemia have usually normal values of morning cortisol concentration, 24-hour cortisol and it is metabolites excretion. However, disturbances of the circadian rhythm of cortisol excretion may occur in these patients. The physiological evening values of cortisolemia should be lower than 50% of morning values. Disturbances of circadian rhythm can manifest in form of slightly increased nocturnal cortisol concentrations – up to borderline values. It was demonstrated that the determination of cortisol concentration at midnight could be useful in testing for subclinical hypercortisolemia. Results =5.4 mg% (150 nmol/l) is recognized as borderline value. Poor arterial hypertension and diabetes control was found in a group classified on the grounds of this criterion [41].
The dexamethasone suppression test is another example of a simple test used in hormonal evaluation of incidentalomas. Its basic version consists of administration of 1 mg of dexamethasone at 11 p.m. followed by measurement of morning serum cortisol concentration at 8 a.m.. Suppression of cortisolemia to a concentration less than 5 mg% (138 nmol/l) is recognized by most of authors as a normal value [42]. This criterion correlates best of all with hypofunction of the other adrenal gland detected on scintigraphy. According to some authors, cortisolemia during short dexamethasone suppression test should not exceed 2 mg%, whereas according to others 3 mg% [10,43]. An improvement in diabetes control after surgery was achieved in a group of patients with subclinical Cushing’s syndrome and diabetes that was classified on the grounds of the strictest criterion of 2 mg% [44,45]. If a lack of suppression is found in tests with 1 mg of dexamethasone, confirmation of tumor autonomization is recommended. A 2-day testing with an administration of 4×2 mg of dexamethasone and an assessment of 24-hour urinary free cortisol excretion (excretion during the second day should decrease below the lower laboratory limit of normal range) should be performed in such cases [28]. Furthermore, determination of low (suppressed) ACTH concentrations and low (as a result of a lack of ACTH stimulation in the cases without autonomous androgen secretion) DHEA and DHEAS concentrations confirms the suspicion of subclinical hypercortisolemia. A secondary hypofunction of the other adrenal gland following an effective operation of the tumor constitutes an ex-post confirmation.
Subclinical Androgen Secretion
Dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulphate (DHEAS) and 17-hydroxyprogesterone (17OHPG) are androgens typically found in the adrenal glands. These androgens are converted to testosterone and dihydrotestosterone. An increased secretion of androgens may indicate malignancy. It should be noted that at least half of adrenal carcinomas follow a course with rapidly increasing cortisolemia and with all the symptoms. Differential diagnosis workup should be carried out for hyperandrogenism in women with incidentaloma taking into account the commonly occurring polycystic ovary syndrome [46]. Recently, a new nosological entity was described for the first time – hyperplasia of the reticular zone that appears in form of tumor with androgen hypersecretion [47].
Asymptomatic Pheochromocytoma
Pheochromocytoma, a tumor that makes up 5% of all incidentaloma cases, is not always the cause of paroxysmal increases in arterial blood pressure. The course of this disease can be accompanied by permanent, slightly increased values of arterial blood pressure, without hypertension or even with orthostatic hypotension [48]. Hypertensive or catecholamine crisis and death can be the first symptoms of this tumor [49]. Clinically silent pheochromocytomas are paradoxically larger in size than tumors producing paroxysmal increases in blood pressure. Abnormal glucose tolerance or diabetes are found in one in three patients with pheochromocytoma [15,20].
In order to evaluate hormonal activity in case of suspected pheochromocytoma, determination of a 24-hour urinary metoxycatecholamine and catecholamine excretion should be performed. This test is more easily available than the determination of catecholamine fraction in the blood, which is recommended by some centers as a slightly more sensitive method. Most patients with pheochromocytoma present with increased serum chromogranin A concentration [50].
Subclinical Hyperaldosteronism
A mild arterial hypertension with a good response to medications can be the only symptom of mineralocorticoid excess. Hypokalemia, which is an element of Conn’s syndrome, may not be present in subclinical primary hyperaldosteronism [10,51]. Determination of plasma renin activity and serum aldosterone concentration are the recommended screening tests. Ratio between aldosterone concentration expressed in ng/dl and plasma renin activity expressed in ng/ml/h of >20 indicates primary hyperaldosteronism. Dynamic tests (e.g. Fludrocortisone suppression, saline infusion or Captopril test) are recommended for confirmation of diagnosis [28,52,53]. A 24-hour urine aldosterone secretion testing is not necessary. An invasive, therefore hazardous, catheterization of both suprarenal veins including the determination of aldosterone concentration gradient, are definitely recommended for confirmation of aldosterone secretion lateralization by most centers [54].
Subclinical Adrenocortical Hypofunction
Bilateral adrenal tumors that raise suspicion of metastases can bring about a subclinical (or undiagnosed for a long time) primary adrenocortical hypofunction. This occurs once at least 90% of active secretory tissue has been damaged. In case of subclinical disease, the presenting signs are increased ACTH and renin concentrations. Then the adrenal reserve (assessed after stimulation with synthetic 1-24ACTH) decreases. The diagnosis of subclinical hypofunction is of importance for surgical planning, as it requires hydrocortisone cover [55].
USG-guided Fine-Needle Aspiration Biopsy
The role of USG-guided fine-needle aspiration biopsy in the diagnostics of adrenocortical tumors is limited and is used only for distinguishing adrenal from extra-adrenal tissue. USG-guided fine-needle aspiration biopsy clearly distinguishes adrenal carcinoma from metastases [56]. However, biopsy is associated with a >3% risk of complications. Catecholamine crisis, caused by a puncture of an undiagnosed pheochromocytoma is the most severe complication [57]. Adrenal carcinoma belongs to a small group of neoplasms that become disseminated by a needle [58]. Thus, following exclusion of pheochromocytoma (hormonal testing, mIBG scintigraphy), adrenal biopsy should be reserved for diagnostic workup of hormonally inactive, high-density tumors that contain no lipids. A preliminary differential diagnosis of nonsecretory adrenal carcinomas and metastases may help identify the origin of metastases and better plan the therapeutic management.
Conclusions
Diagnostic procedure carried out after incidental detection of an adrenal tumor must provide answers as to whether surgery or observation is indicated.
Malignancy or hormonal activity of the lesion are indications for resection. Tumors presenting with inhomogeneity, high density, slow contrast washout on CT, low lipid content or lack of lipids on MRI raise suspicion of malignancy. Surgery should also be considered for large-sized tumors – a limit of 4 cm seems to be the safest one. In cases with inhomogeneous, lipid-free lesions with high density, a clinically silent pheochromocytoma should be considered. This suspicion is confirmed by increased excretion of 24-hour urinary catecholamines and their metabolites, increased catecholamine concentration in the blood and increased tracer uptake on 123I mIBG or somatostatin receptor scintigraphy.
Other syndromes caused by adrenal incidentalomas include subclinical hypercortisolemia and subclinical hyperaldosteronism. Subclinical primary adrenocortical insufficiency with decreased adrenal reserve is found in cases with bilateral metastases.
Observation, repeated imaging and hormonal tests are necessary, even in cases when the tumor does not require surgical management. Recommended follow-up visits should take place at 6, 12 and 24 months after detection of lesion. Follow-up examination at 3 months is indicated in case of young patients or tumors with borderline size. Tumor growth by more than 0.8 cm within a year is an indication for surgery.
References
- 1.Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29:298–302. doi: 10.1007/BF03344099. [DOI] [PubMed] [Google Scholar]
- 2.Song JH, Chaudhry FS, Mayo-Smith WW, et al. The Incidental Adrenal Mass on CT: Prevalence of Adrenal Disease in 1,049 Consecutive Adrenal Masses in Patients with No Known Malignancy. Am J Roentgenol. 2008;190:1163–68. doi: 10.2214/AJR.07.2799. [DOI] [PubMed] [Google Scholar]
- 3.Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40:80–83. doi: 10.1007/s12020-011-9445-6. [DOI] [PubMed] [Google Scholar]
- 4.Hornsby PJ. Ageing of the Human Adrenal Cortex. Sci Aging Knowl Environ. 2004;35:1–8. doi: 10.1126/sageke.2004.35.re6. [DOI] [PubMed] [Google Scholar]
- 5.Dobble JW. Adrenocortical nodular hyperplasia: The aging adrenal. J Pathol. 1969;99:1–18. doi: 10.1002/path.1710990102. [DOI] [PubMed] [Google Scholar]
- 6.Hedeland H, et al. On the prevalence of Adrenocortical adenomas in an autopsy material in relation to hypertension and diabetes. ActaMed Scand. 1968;184(3):211–14. doi: 10.1111/j.0954-6820.1968.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 7.Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inaparrent adrenal mass („IncIdentaloma”) Ann Intern Med. 2003;138:424–29. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 8.Young WF. Management approaches to adrenal incidentalomas: a view from Rochester, Minnesota. Endocrinol Metab Clin North Am. 2000;29:159–85. doi: 10.1016/s0889-8529(05)70122-5. [DOI] [PubMed] [Google Scholar]
- 9.Barzon L, Sonino N, Fallo F. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149:273–85. doi: 10.1530/eje.0.1490273. [DOI] [PubMed] [Google Scholar]
- 10.Zeiger MA, Siegelman SS, Hamrahian AH. Medical and surgical evaluation and treatment of adrenal incidentalomas. J Clin Endocrinol Metab. 2011;96(7):2004–15. doi: 10.1210/jc.2011-0085. [DOI] [PubMed] [Google Scholar]
- 11.Günther RW, Kelbel C, Lenner V. Real-time ultrasound of normal adrenal glands and small tumors. J Clin Ultrasound. 1984;12(4):211–17. doi: 10.1002/jcu.1870120408. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich CF, Wehrmann T, Hoffmann C, et al. Detection of the adrenal glands by endoscopic or transabdominal ultrasound. Endoscopy. 1997;29(9):859–64. doi: 10.1055/s-2007-1004322. [DOI] [PubMed] [Google Scholar]
- 13.Trojan J, Schwarz W, Sarrazin C, et al. Role of ultrasonography in the detection of small adrenal masses. Ultraschall Med. 2002;23(2):96–100. doi: 10.1055/s-2002-25190. [DOI] [PubMed] [Google Scholar]
- 14.Hsu-Chong Y. Sonography of the Adrenal Glands: Normal Glands and Small Masses. Am J Roentgenol. 1980;135:1167–77. doi: 10.2214/ajr.135.6.1167. [DOI] [PubMed] [Google Scholar]
- 15.Mansmann G, Lau J, Balk E, et al. The Clinically Inapparent Adrenal Mass: Update In Diagnosis and Management. Endocrine Rev. 2004;25:309–40. doi: 10.1210/er.2002-0031. [DOI] [PubMed] [Google Scholar]
- 16.Schteingart DE, Doherty GM, Gauger PG, et al. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer. 2005;12:667–80. doi: 10.1677/erc.1.01029. [DOI] [PubMed] [Google Scholar]
- 17.Nawar R, Aron D. Adrenal incidentalomas – a continuing management dilemma. Endocr Relat Cancer. 2005;12:585–98. doi: 10.1677/erc.1.00951. [DOI] [PubMed] [Google Scholar]
- 18.Thompson GB, Young WF., Jr Adrenal incidentaloma. Curr Opin Oncol. 2003;15:84–90. doi: 10.1097/00001622-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Ilias I, Sahdev A, Reznek R, et al. The optimal imaging of adrenal tumors: a comparison of different methods. Endocr Relat Cancer. 2007;14:587–99. doi: 10.1677/ERC-07-0045. [DOI] [PubMed] [Google Scholar]
- 20.Blake MA, Kalra MK, Maher MM, et al. Pheochromocytoma: An Imaging Chameleon. Radio Graphics. 2004;24:87–99. doi: 10.1148/rg.24si045506. [DOI] [PubMed] [Google Scholar]
- 21.Stajgis M, Guzikowska-Ruszkowska I, Horst-Sikorska W, et al. CT diagnostic imaging of adrenal adenomas. Pol J Radiol. 2005;70(2):62–68. [Google Scholar]
- 22.Adusumilli S, Ramchandani P. Adrenal Myelolipoma” eMedicine – Radiology online. www.emedicine.com/radio/topic18.htm.
- 23.Cyran KM, Kenney PJ, Memel DS, et al. Adrenal myelolipoma. Am J Roentgenol. 1996;166(2):395–400. doi: 10.2214/ajr.166.2.8553954. [DOI] [PubMed] [Google Scholar]
- 24.Pantalone KM, Gopan T, Remer EM, et al. Change in adrenal mass size as a predictor of a malignant tumor. Endocr Pract. 2010;16:577–87. doi: 10.4158/EP09351.OR. [DOI] [PubMed] [Google Scholar]
- 25.Weir J, Abrahams P. Atlas Obrazowy Anatomii Człowieka. Polish edition. Urban & Partner; Wrocław: 2005. Wstęp – Tomografia Komputerowa; pp. IX–XI. [in Polish] [Google Scholar]
- 26.Podgórska J, Cieszanowski A, Bednarczuk T. Adrenal imaging. Pol J Endocrinol. 2012;63(1):71–81. [PubMed] [Google Scholar]
- 27.Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: current status. Am J Roentgenol. 2002;179:559–68. doi: 10.2214/ajr.179.3.1790559. [DOI] [PubMed] [Google Scholar]
- 28.Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:601–10. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 29.Thompson GB, Young WF. Adrenal incidentaloma. Curr Opin Oncol. 2003;15:84–90. doi: 10.1097/00001622-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Zielonko J, Studniarek M, Rzepko R, et al. Value of MRI in differentiating adrenal masses: Quantitative analysis of tumor signal intensity. Pol J Radiol. 2008;73(2):7–12. [Google Scholar]
- 31.Bravo EL, Tagle R. Pheochromocytoma: state-of-the-art and future prospects. Endocr Rev. 2003;24:539–53. doi: 10.1210/er.2002-0013. [DOI] [PubMed] [Google Scholar]
- 32.van der Harst E. (123)I]metaiodobenzylguanidine and (111)In]octreotide uptake in benign and malignant pheochromocytomas. J Clin Endocrinol Metab. 2001;86:685–93. doi: 10.1210/jcem.86.2.7238. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson B, Orlefors H, Oberg K, et al. Developments in PET for the detection of endocrine tumours. Tumour Biol. 2005;19:311–24. doi: 10.1016/j.beem.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Mann GN, Jeanne ML, Pham P. (11)C]metahydroxyephedrine and (18)f]fluorodeoxyglucose positron emission tomography improve clinical decision making in suspected pheochromocytoma. Ann Surg Oncol. 2006;13:187–97. doi: 10.1245/ASO.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Alencar GA, Barisson Villares Fragoso MC, Itaya Yamaga L, et al. Image in Endocrinology: 18F-FDG-PET/CT Imaging of ACTH-Independent Macronodular Adrenocortical Hyperplasia (AIMAH) Demonstrating Increased 18F-FDG Uptake. J Clin Endocrinol Metab. 2011;96:3300–1. doi: 10.1210/jc.2011-1397. [DOI] [PubMed] [Google Scholar]
- 36.Rossi R, Tauchmanova L, Luciano A, et al. Subclinical Cushing’s Syndrome in Patients with Adrenal Incidentaloma: Clinical and Biochemical Features. J Clin Endocrinol Metab. 2000;85:1440–48. doi: 10.1210/jcem.85.4.6515. [DOI] [PubMed] [Google Scholar]
- 37.Tauchmanovà L, Rossi R, Biondi B, et al. Patients with Subclinical Cushing’s Syndrome due to Adrenal Adenoma Have Increased Cardiovascular Risk. J Clin Endocrinol Metab. 2002;87:4872–78. doi: 10.1210/jc.2001-011766. [DOI] [PubMed] [Google Scholar]
- 38.Chiodini I, et al. Bone Involvement in Eugonadal Male Patients with Adrenal Incidentaloma and Subclinical Hypercortisolism. J Clin Endocrinol Metab. 2002;87:5491–94. doi: 10.1210/jc.2002-020399. [DOI] [PubMed] [Google Scholar]
- 39.Terzolo M, Pia A, Alì A, et al. Adrenal Incidentaloma: A New Cause of the Metabolic Syndrome? J Clin Endocrinol Metab. 2002;87:998–1003. doi: 10.1210/jcem.87.3.8277. [DOI] [PubMed] [Google Scholar]
- 40.Erbil Y, Ademoğlu E, Ozbey N, et al. Evaluation of the cardiovascular risk in patients with subclinical Cushing syndrome before and after surgery. World J Surg. 2006;30:1665–71. doi: 10.1007/s00268-005-0681-x. [DOI] [PubMed] [Google Scholar]
- 41.Terzolo M, Bovio S, Pia A, et al. Midnight serum cortisol as a marker of increased cardiovascular risk in patients with a clinically inaparrent adrenal adenoma. Eur J Endocrinol. 2005;153:307–15. doi: 10.1530/eje.1.01959. [DOI] [PubMed] [Google Scholar]
- 42.Tsagarakis S, Vassiliadi D, Thalassinos N. Endogenous subclinical hypercortisolism: diagnostic uncertainties and clinical implications. J Endocrinol Invest. 2006;29:471–82. doi: 10.1007/BF03344133. [DOI] [PubMed] [Google Scholar]
- 43.Catargi B, Rigalleau V, Poussin A, et al. Occult Cushing’s Syndrome in Type-2 Diabetes. JCEM. 2003;88:5808–13. doi: 10.1210/jc.2003-030254. [DOI] [PubMed] [Google Scholar]
- 44.Leibowitz G, Tsur A, Chayen SD. Pre-clinical Cushing’s syndrome: an unexpected frequent cause of poor glycaemic control in obese diabetic patients. Clin Endocrinol (Oxf) 1996;44:717–22. doi: 10.1046/j.1365-2265.1996.737558.x. [DOI] [PubMed] [Google Scholar]
- 45.Midorikawa S, Sanada H, Hashimoto S, et al. The improvement of insulin resistance in patients with adrenal incidentaloma by surgical resection. Clin Endocrinol (Oxf) 2001;54:797–804. doi: 10.1046/j.1365-2265.2001.01274.x. [DOI] [PubMed] [Google Scholar]
- 46.Kasperlik-Załuska A. Przypadkowo wykryte guzy nadnerczy – zasady postępowania. Endokrynol Pl. 2002;53(Supl.1/2):88–92. [in Polish] [Google Scholar]
- 47.Ghayee HK, Rege J, Watumull LM, Nwariaku, et al. Clinical, biochemical, and molecular characterization of macronodular adrenocortical hyperplasia of the zona reticularis: a new syndrome. J Clin Endocrinol Metab. 201;96(2):243–50. doi: 10.1210/jc.2010-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasperlik-Załuska A, Rosłonowska E, Słowinska-Srzednicka J, et al. 1,111 Patients with Adrenal Incidentalomas Observed at a Single Endocrinological Center. Incidence of Chromaffin Tumors. Ann NY Acad Sci. 2006;1073(1):38–46. doi: 10.1196/annals.1353.004. [DOI] [PubMed] [Google Scholar]
- 49.Sutton MG, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma: review of a 50-year autopsy series. Mayo Clin Proc. 1981;56:354–60. [PubMed] [Google Scholar]
- 50.Sowers KM, Sowers JR. Manual of Endocrinology and Metabolism. Lipincott W&W; 2002. Pheochromocytomas; pp. 139–43. [Google Scholar]
- 51.Rossi GP, Bernini G, Caliumi C, et al. A Prospective Study of the Prevalence of Primary Aldosteronism in 1,125 Hypertensive Patients. J Am Coll Cardiol. 2006;48:2293–300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 52.National Institutes of Health. Management of the Clinically Inapparent Arenal Mass (Incidentaloma. Final Statement. July2002 [Google Scholar]
- 53.Sonoyama T, Sone M, Miyashita K, et al. Significance of adrenocorticotropin stimulation test in the diagnosis of an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2011;96(9):2771–78. doi: 10.1210/jc.2011-0573. [DOI] [PubMed] [Google Scholar]
- 54.Harvey A, Pasieka JL, Kline G, et al. Modification of the protocol for selective adrenal venous sampling results in both a significant increase in the accuracy and necessity of the procedure in the management of patients with primary hyperaldosteronism. Surgery. 2012;152(4):643–49. doi: 10.1016/j.surg.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Lutz A, Stojkovic M, Schmidt M, et al. Adrenocortical function in patients with macrometastases of the adrenal gland. Eur J Endocrinol. 2000;143(1):91–97. doi: 10.1530/eje.0.1430091. [DOI] [PubMed] [Google Scholar]
- 56.Harisinghani MG, Maher MM, Hahn PF, et al. Predictive value of benign percutaneous adrenal biopsies in oncology patients. Clin Radiol. 2002;57:898–901. doi: 10.1053/crad.2002.1054. [DOI] [PubMed] [Google Scholar]
- 57.Casola G, Nicolet V, vanSonnenberg E. Unsuspected phaeochromocytoma: risk of blood-pressure alterations during percutaneous adrenal biopsy. Radiology. 1986;159:733–35. doi: 10.1148/radiology.159.3.3517958. [DOI] [PubMed] [Google Scholar]
- 58.Welch TJ, Sheedy PF, II, Stephens DH, et al. Percutaneous adrenal biopsy: review of a 10-year experience. Radiology. 1994;193:341–44. doi: 10.1148/radiology.193.2.7972740. [DOI] [PubMed] [Google Scholar]