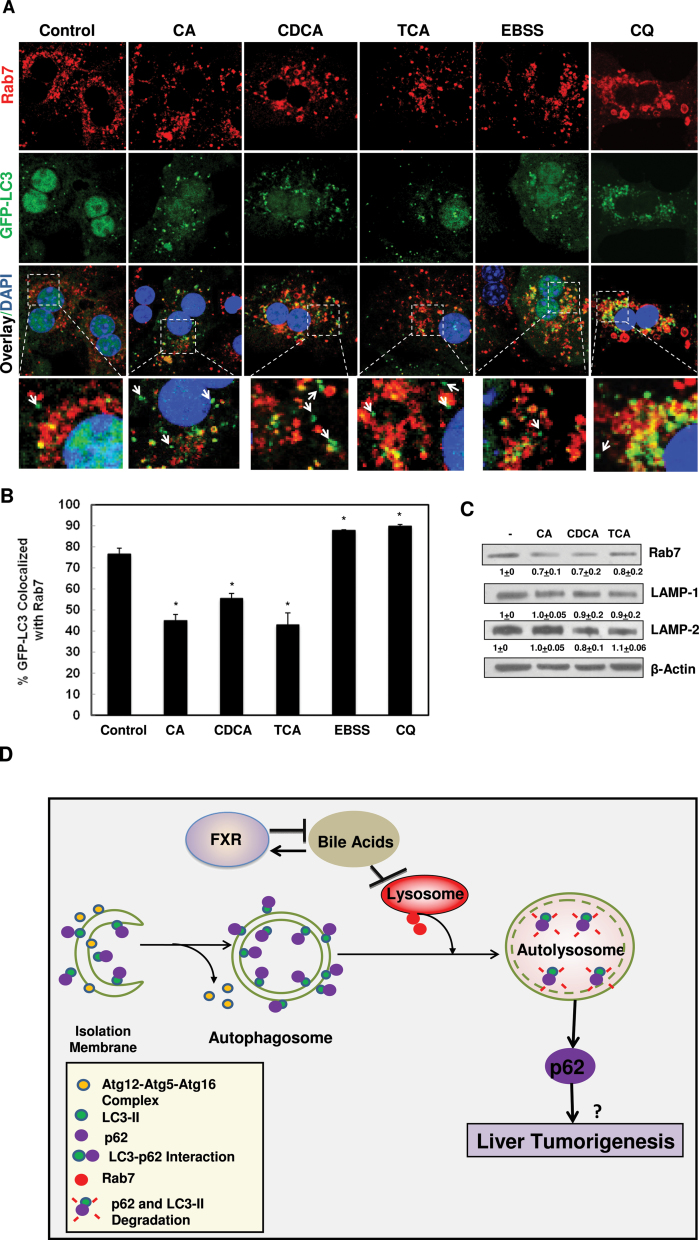
FIG. 7.
BAs decreased the colocalization of GFP-LC3 with Rab7. A, Primary hepatocytes were infected with adenovirus GFP-LC3 (100 viral particles per cell) overnight and then treated with BAs, CQ or EBSS for 6h. Cells were then immunostained with Rab7 followed by confocal microscopy. Representative images are shown, and the lower panels are enlarged images from the boxed areas. Arrows: GFP-LC3 puncta only. B, Percentage of GFP-LC3 colocalized with Rab7 was quantified (>20 cells were counted in each experiment from at least three independent experiments). C, Hepatocytes were treated with BAs for 6h and total cell lysates were subjected to immunoblot analysis followed by densitometry analysis as described in Figure 1A (n = 3). D, A proposed model that BAs inhibit autophagic flux by suppressing Rab7-mediated autophagsomal-lysosomal fusion in hepatocytes. During autophagy induction, small pieces of isolation membranes grow to form double-membrane autophagosomes, a process that is regulated by Atg5-Atg12-Atg16 complex and LC3-PE conjugation (LC3-II). LC3 also directly interacts with autophagy substrate protein p62 and recruit p62 into autophagosomes. Autophagosomes then fuse with lysosomes to form autolysosomes, where LC3-II and p62 are degraded. This process is mediated by Rab7 and other fusion proteins on either autophagosomes or lysosomes. BAs activate FXR whereas activated FXR negatively regulates synthesis of hepatic BAs. BAs inhibit the fusion of autophagosomes with lysosomes in hepatocytes likely due to decreased expression of Rab7 and its targeting to autophagosomes, resulting in impaired autophagic flux. Impaired autophagic flux leads to the accumulation of LC3-II and p62. Accumulated p62 may contribute to liver tumorigenesis.