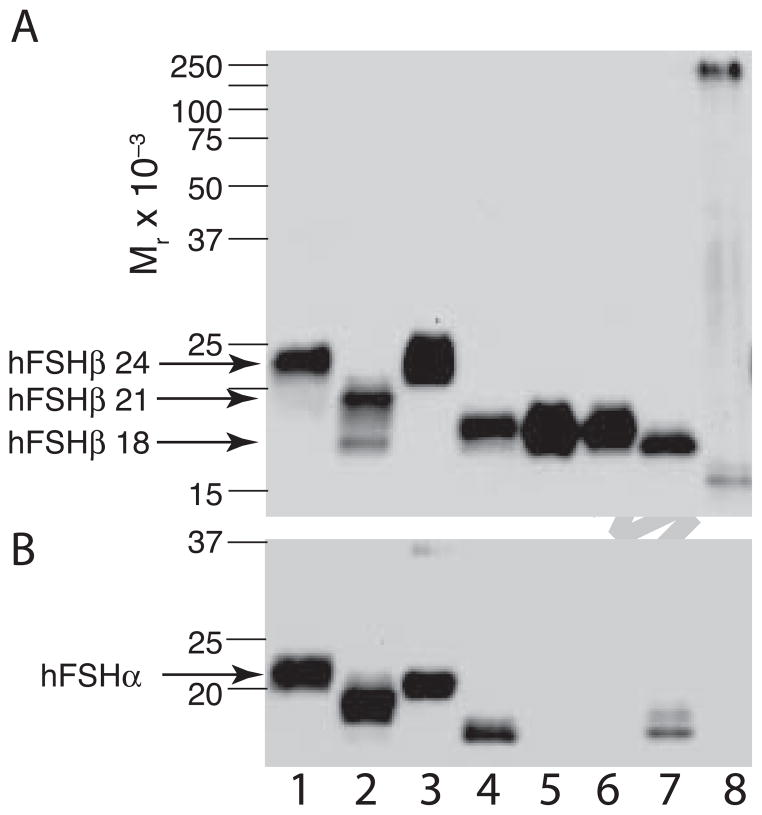
Figure 6. Comparison of recombinant insect hFSH subunit electrophoretic mobilities with those of pituitary FSH glycoforms.
A. FSHβ-Western blot comparing mobilities of hFSH glycoforms with those of recombinant insect cell hFSHβ subunit preparations. The primary antibody was RFSH20. B. FSHα-Western blot using HT13 primary antibody. The same preparations were compared in both blots. Lane 1, pituitary hFSH (AFP4161B); lane 2, hypo-glycosylated hFSH; lane 3, fully-glycosylated hFSH; lane 4, wt recombinant insect hFSH; lane 5, αT54A mutant recombinant insect hFSH; lane 6, αT80A mutant recombinant insect hFSH; lane 7, βT26A mutant recombinant insect hFSH; lane 8, recombinant bacterial hFSHβ. Pre-stained BioRad MW marker positions are indicated by lines.