Abstract
Extraction of carbon nanotubes (CNTs) from biological matrices such as rat lung tissue is integral to developing a quantification method for evaluating the environmental and human health exposure and toxicity of CNTs. The ability of various chemical treatment methods, including Solvable (2.5% sodium hydroxide/surfactant mixture), ammonium hydroxide, nitric acid, sulfuric acid, hydrochloric acid, hydrofluoric acid, hydrogen peroxide, and proteinase K, to extract CNTs from rat lung tissue was evaluated. CNTs were quantified using programmed thermal analysis (PTA). Two CNTs were used to represent the lower (500°C) and upper (800°C) PTA limit of CNT thermal stability. The recovery efficiency of each of the eight chemical reagents evaluated was found to depend on the ability to (1) minimize oxidation of CNTs, (2) remove interfering background carbon from the rat lung tissue, and (3) separate the solid-phase CNTs from the liquid-phase dissolved tissue via centrifugation. A two-step extraction method using Solvable and proteinase K emerged as the optimal approach, enabling a recovery of 98 ± 15% of a 2.9 ± 0.19 µg CNT loading that was spiked into whole rat lungs. Due to its high yield and applicability to low organ burdens of nanomaterials, this extraction method is particularly well suited for in vivo studies to quantify clearance rates and retained CNTs in lungs and other organs.
Keywords: Carbon nanotube, rat lung, extraction, digestion, quantification
Carbon nanotubes (CNTs) are steadily becoming a viable component in consumer products, drug-delivery systems, composite materials, and electronics.1 The increased demand and production of CNTs warrants consideration of their risks to the environment and human health as a consequence of exposure throughout product life. Inhalation and dermal exposures during CNT manufacturing are of great concern; inhalation is the most likely exposure pathway.2–5 Rats are often used as model organisms for in vivo studies of respiratory tract responses to CNTs. Many studies have explored the pulmonary toxicity of CNTs,6–11 but little is known about exposure-dose-response relationships due to technical difficulties in reliably extracting and detecting CNTs from target tissues and complex matrices.12
In a previous study, we showed that CNTs can be quantified in complex organic matrices using programmed thermal analysis (PTA),13 an analytical method that relies on the unique thermal stability of CNTs. PTA uses a similar instrument setup as the National Institute for Occupational Safety and Health (NIOSH) 5040 method,14 which is designed for elemental and organic carbon analysis, but it is designed specifically for CNTs.13 The PTA instrument detection limit for carbon mass is approximately 200 ng. In this study, PTA was used to evaluate the recovery and thermal stability of CNTs. We used two types of multi-walled CNTs (MWCNTs) to represent the lower and upper bounds of CNT thermal stability, as determined previously.13 The thermal fingerprint (thermogram) of the CNTs obtained from PTA was used to indicate changes in the thermal stability of the CNTs after extraction. As a complementary analysis, Raman spectroscopy was used to determine if CNT structural damage was evident; such damage may indicate a change in the thermal stability.15
Chemical digestion methods utilizing oxidants, acids, and alkalis are commonly used to extract metals from soils, sediments, sludges, plants, and animal tissues.16, 17 For CNTs in complex matrices, the major challenge is dissolving the matrix carbon while keeping the CNTs intact, which is required for quantification. Alkalis and enzyme reagents effectively dissolve lung tissue while minimizing damage to soot carbon,18, 19 but they have not yet been applied to CNTs. Although CNTs are one of the most thermodynamically stable forms of carbon, these digestion reagents (e.g., HNO3) may induce defects (i.e., break CNT carbon-carbon bonds, oxidize CNT surface) or degrade them to carbon monoxide and carbon dioxide.20–22 Increasing the number of defects in the CNT decreases the thermal stability, which makes detection of CNTs over background carbon more difficult when using PTA. So, developing an extraction method that minimizes physical and chemical changes to the CNT is necessary for obtaining a high CNT recovery.
The aim of this study was to develop a method for extracting CNTs from rat lung tissue for CNT quantification analysis. To achieve this, we examined (1) the effect of various chemical digestion reagents on the recoverability of CNTs as defined by PTA, (2) the ability of various chemical digestion reagents to completely dissolve rat lung tissue, and (3) the ability to extract CNTs from rat lung tissue using the optimal digestion method defined by (1) and (2). Commonly used acids, alkalis, oxidants, and enzymes were evaluated as possible chemical digestion reagents. We considered the digestion reagent to be unsuccessful if it (a) damaged/destroyed the CNTs to the extent of impacting quantification or (b) did not effectively dissolve the rat lung tissue. The results of this study will have an impact on carbon nanomaterial toxicology studies, specifically those aiming to identify tissue doses that are related to adverse outcomes, and they will expand our knowledge about carbon nanomaterial extraction from complex matrices.
RESULTS AND DISCUSSION
Effect of Chemical Treatment Methods on CNTs
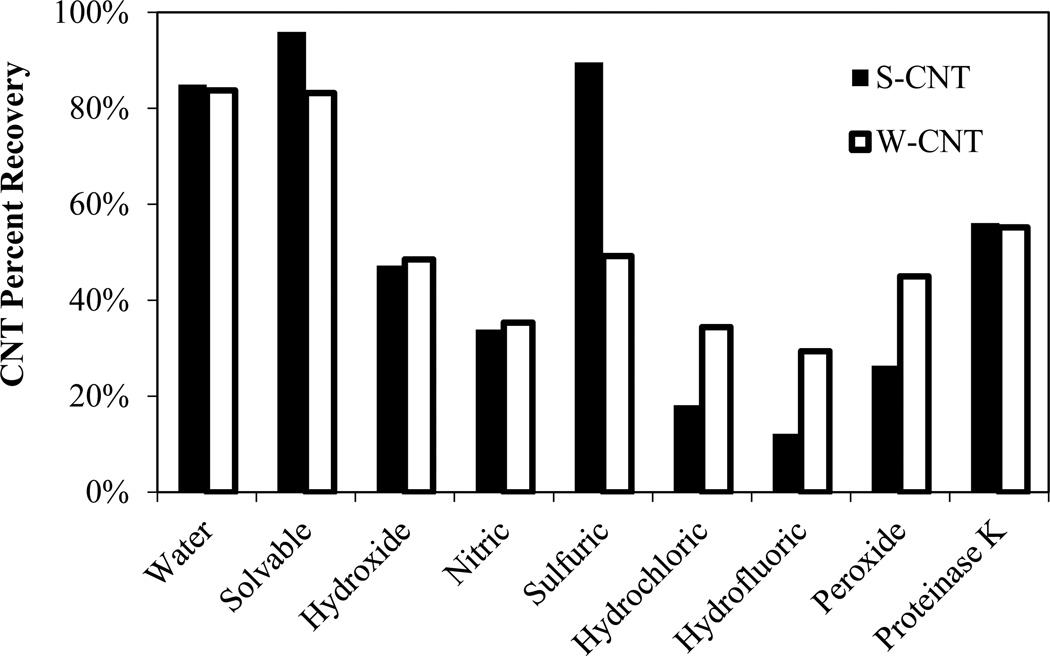
Figure 2 shows the percent recovery for strong (S-CNT) and weak (W-CNT) CNTs after treatment with various reagents. Solvable resulted in the highest recovery for both CNT types. For some of the extraction methods, recovery >60% was not achieved because the CNTs could not form a stable pellet, resulting in losses during washing, or because they adhered to the sides of the centrifuge vials. Hydrogen peroxide and hydrochloric acid samples stuck to the centrifuge vials (e.g., Figure SI-2) and did not form a stable pellet, resulting in a significant loss of CNTs. Hydrofluoric acid, proteinase K, and water samples stuck slightly to the vials, and they formed semi-stable (not fully compacted) pellets. Solvable and ammonium hydroxide samples formed very solid pellets without any sticking to the vials, although pellet formation decreased for hydroxide samples as the number of washing steps increased. For the samples that did not produce compact pellets, neither increasing the centrifugation speed (up to 25,000 × g) nor increasing the time (up to 1 hr) improved pellet formation. A functionalized version of the W-CNT was used to determine the applicability of centrifugation using Solvable, the most optimal method. Even though the functionalized W-CNTs are very stable in water, pellet formation was compact (e.g., Figure SI-3), indicating the effectiveness of using Solvable for hydrophilic CNTs as well. One explanation for this observation is that Solvable contains surfactants, which presumably could keep CNTs from sticking to the centrifuge tube, as well as hydroxyl ions, which could promote aggregation through electric double layer compression. Because of problems with pellet formation during the separation step, the recovery results intrinsically reflect both separation and the damage done by each method. Consequently, other analyses, such as thermal analysis and Raman spectroscopy, were completed to clarify the recovery results.
Figure 2.
Recovery for S-CNTs and W-CNTs after treatment with the chemical digestion reagents (single run as a screening experiment).
The PTA thermograms of the CNT samples (see SI, Figure SI-4) were analyzed to determine if the reagents had an effect on the CNT oxidation temperature. This is important for two reasons: (1) a significant decrease in oxidation temperature may indicate CNT mass loss from chemical oxidation during the extraction process, and (2) decreasing the oxidation temperature of the CNTs results in an increase in possible interference with matrices containing background carbon that has similar oxidation temperatures.
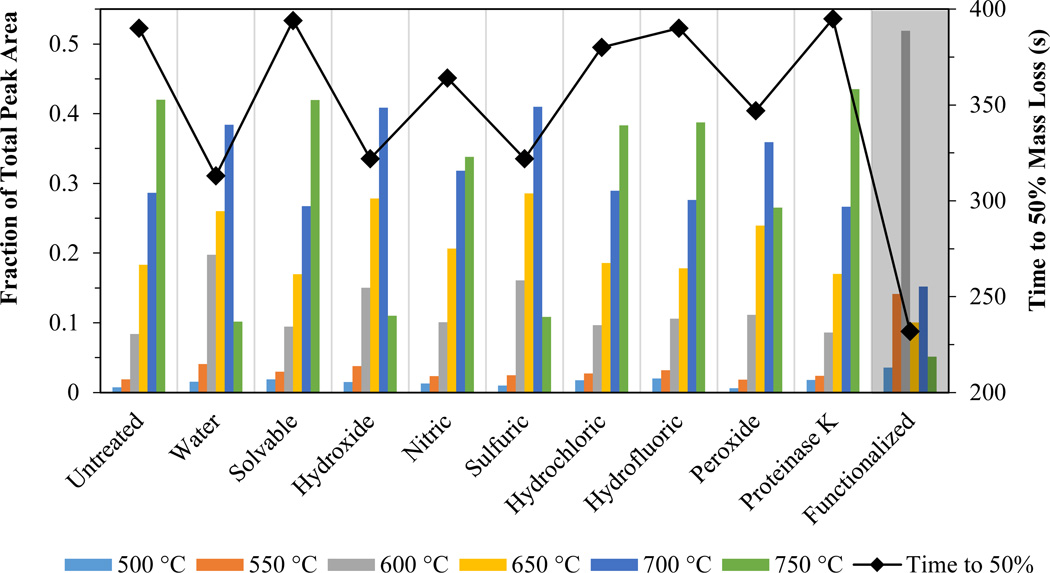
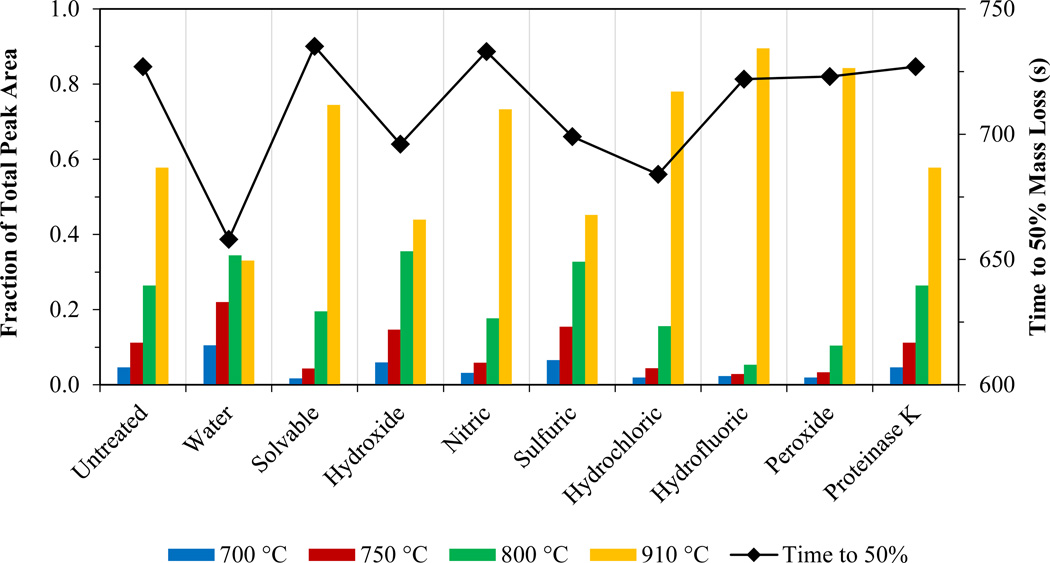
The thermograms had eight distinct peaks that correlated to the temperature program steps (500, 550, 600, 650, 700, 750, 800, and 910°C; see SI, Figures SI-1 and SI-4). To analyze thermogram changes resulting from the extraction process, the area of each peak was normalized to the total thermogram area (peak fraction = peak area/total area). Figures 3 and 4 show the peak fraction of the total CNT area for each temperature step and the time required for 50% mass loss after treatment of the W-CNTs and the S-CNTs, respectively, with various methods. A decrease in the time to 50% mass loss indicates a decrease in the thermal strength of the CNT. For both CNTs, the “untreated” sample (i.e., the raw CNT analyzed as a dry powder) was used as a control.
Figure 3.
Peak fraction of the total CNT area for six temperature steps (bars) and time required for 50% mass loss (solid diamonds connected by a line) for a W-CNT under oxidizing conditions after treatment with the chemical digestion reagents. Grey shaded area is the functionalized W-CNT after treatment with Solvable.
Figure 4.
Peak fraction of the total CNT area for four temperature steps (bar) and time required for 50% mass loss (solid diamonds connected by a line) for an S-CNT under oxidizing conditions after treatment with the chemical digestion reagents.
W-CNTs evolved over a wide temperature range from 500 to 750°C, with the maximum peak fraction occurring at 750°C for the untreated sample. Samples treated with Solvable, hydrochloric acid, hydrofluoric acid, and proteinase K had no change in the time to 50% mass loss (<5%), indicating that these reagents had little effect on the thermal stability. Nitric acid had a mild effect (5–10%), and the peak fraction at 750°C decreased but remained the maximum in comparison with the other peak fractions. Water, hydroxide, sulfuric acid, and peroxide each had a significant effect on the time to 50% mass loss (10–20%), and the maximum peak fraction temperature decreased to 700°C. The functionalized W-CNT treated with Solvable had a severe decrease of 40%, and the maximum peak fraction temperature decreased to 600°C.
S-CNTs evolved from 700 to 910°C; the maximum peak fraction occurred at 910°C for the untreated sample. Solvable, hydroxide, nitric acid, sulfuric acid, hydrofluoric acid, peroxide, and proteinase K had no change in the time to 50% mass loss (<5%). Water and hydrochloric acid had a mild change (5–10%). These results indicate the S-CNTs’ ability to withstand oxidation caused by the reagents used in the extraction process.
The key factor in the thermal stability was the availability of oxygen on the surface of the CNT. For both CNT types, the “water” sample exhibited the largest decrease in thermal stability. This was attributed to the CNT dispersant, which likely remained wrapped around the CNTs during washing and then contributed oxygen during the thermal analysis, causing premature oxidation. This is especially evident for the S-CNTs that were resilient to oxidation from harsh reagents but exhibited a decrease in the time to 50% mass loss for the water sample when the dispersant was presumably still present on the CNTs. For the other reagents, the dispersant was likely destroyed (i.e., oxidized, hydrolyzed) during the treatment process and did not contribute oxygen. Similarly, the functionalized W-CNT, which contained a large amount of oxygenated functional groups (~6%), exhibited the most significant change in thermal stability compared to W-CNTs that had gone through the same treatment process.
No reagent was detrimental (>30% change) to the thermal stability of either CNT types. Peroxide, hydroxide, and sulfuric were the most damaging to the W-CNTs, and the reagents had little effect on the thermal stability S-CNTs, which is in good agreement with their “weak/strong” classifications. For nitric, hydrofluoric, and hydrochloric, significant CNT damage was not observed from the thermograms (i.e., >10% change to the time to 50% mass loss), but the percent recovery was low in comparison to the other reagents (Figure 2). This was attributed to the poor performance of the centrifugal separation method and indicates that new separation methods need to be developed if these reagents are to be used for applications requiring different reagents (e.g., hydrofluoric acid for dissolving silica in sediments). Furthermore, some of the treatments resulted in damage to the PTA instrument tubing (e.g., silicon, copper) and metal parts (e.g., stainless steel in valves and FID) from residual acids/alkalis (e.g., sulfuric acid, nitric acid). Although additional washing steps could minimize this risk, this may result in decreased CNT recovery.
Evaluating CNT Structural Damage Using Raman Spectroscopy
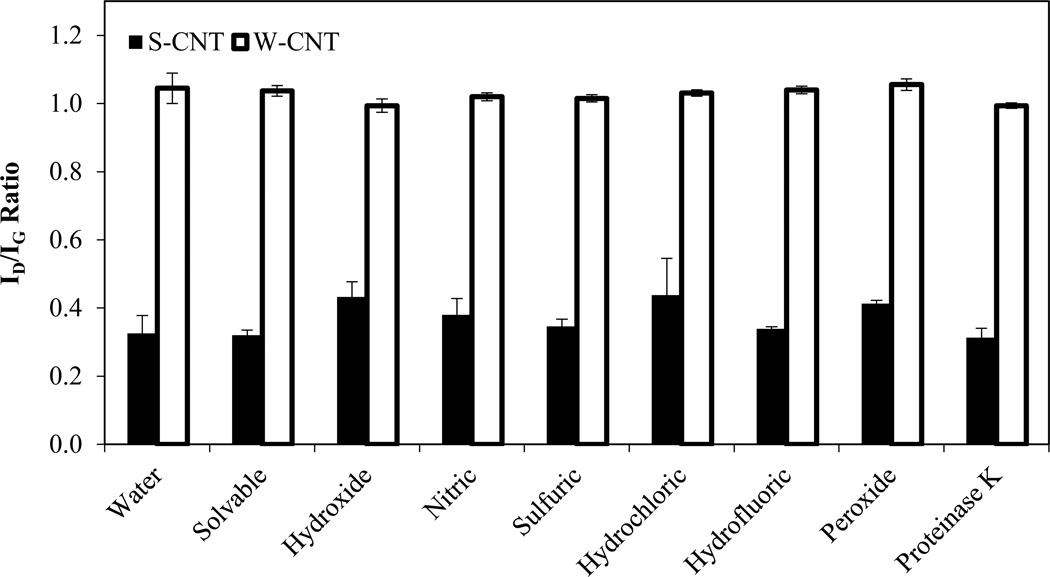
Raman spectroscopy is a good characterization tool for CNTs with spectral peaks that are specific to CNTs or graphite and to no other form of carbon.33 Figure 5 shows the ID/IG ratios for S-CNTs and W-CNTs after treatment with the chemical digestion reagents. For the S-CNTs, hydroxide, nitric, hydrochloric, and peroxide each exhibited a >20% increase in the ID/IG ratio compared to water only, but this amount of damage was not enough to have a marked effect on the thermal stability as revealed during thermal analysis. For example, hydroxide increased the ID/IG ratio from 0.33 ± 0.05 to 0.43 ± 0.04, but the time to 50% mass loss decreased only 4%. This suggests that defects alone are not enough to reduce the thermal stability of the CNT, and that oxygenated groups must be present at these defect sites. For the W-CNTs, no significant change (<5%) in the ID/IG ratio was observed for any of the treatment methods. This absence of change is expected because the W-CNTs already have a high initial defect density, which leaves little room for the formation of additional defects.34 Although there was no change in the defect density of the W-CNTs after treatment, some reagents caused a decrease in the thermal stability (e.g., peroxide, sulfuric). This suggests that the decrease in thermal stability for W-CNTs was caused by the addition of oxygenated groups and not new defects.
Figure 5.
ID/IG ratios obtained from Raman spectroscopy for CNTs after treatment with the chemical digestion reagents (60°C for 24 hrs). Error bars represent standard deviation for triplicate samples.
Rat Lung Tissue Digestion
For CNT quantification, the rat lung tissue should be fully dissolved, as its remaining carbon can interfere with CNT detection using PTA. When dissolving organic matter biological tissue with acids, alkalis, oxidants, or enzymes, generally some thermal energy beyond room temperature is needed to speed up the process. The boiling point of the liquid (i.e., temperature at reflux) generates the highest activity while keeping the sample contained; this can range from high (e.g., 337°C for H2SO4) to low (e.g., 84°C for HCl) temperatures. However, at higher temperatures, controlling the activity of the treatment becomes more difficult (i.e., the reaction occurs too fast), and CNTs may oxidize. In this study, chemical treatment of both CNT types at the reagent reflux temperatures resulted in major CNT loss when using strong acids and oxidants (e.g., H2SO4, H2O2; results not shown, recovery less than 10%). As a result, the rat lung tissue treatment methods were evaluated at a milder treatment temperature of 60°C.
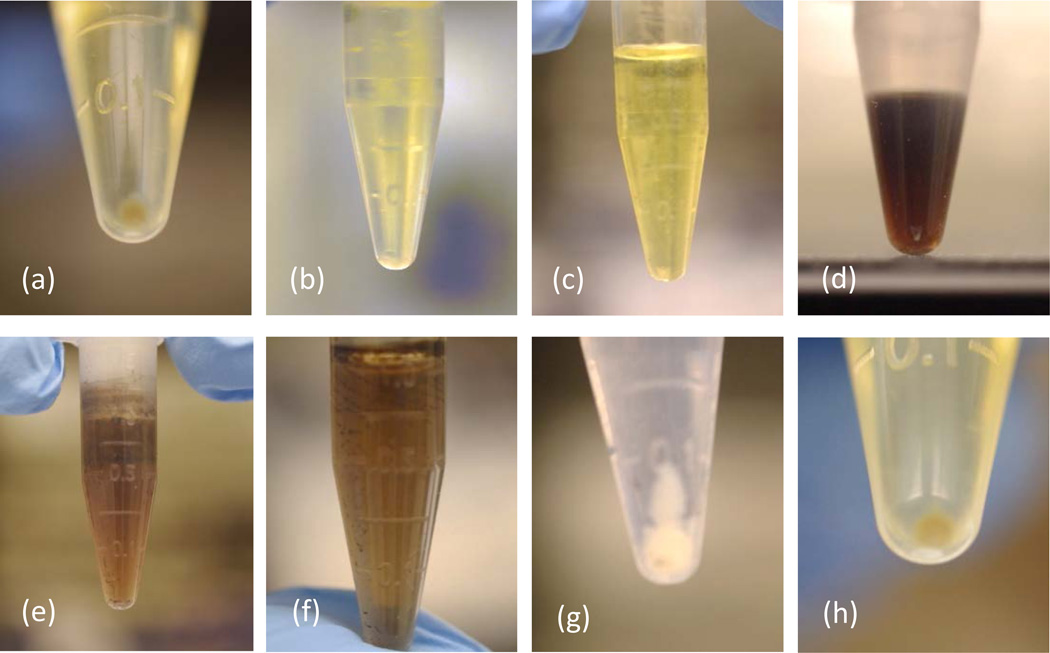
For the rat lung tissue, initial success was assessed visually; samples that were dissolved (i.e., no visible tissue remaining) were considered successful, whereas those with tissue remaining were deemed unsuccessful (Figure 6). All alkalis (Solvable, ammonium hydroxide, tetramethylammonium hydroxide (TMAH) (not shown), and potassium hydroxide/ethanol (KOH-EtOH, not shown)) effectively dissolved the tissue within the first hour. However, KOH-EtOH and TMAH are not discussed further because KOH-EtOH melted the QFFs during PTA, and upon heating TMAH produced triethylamines, which makes its use non-ideal due to the offensive odor that is produced. The resultant liquid for the alkalis was transparent yellow, and a small pellet of non-digestible carbon was formed (Figures 6a and 6b). Nitric acid effectively dissolved the tissue within 30 minutes, resulting in a transparent yellow solution with no obvious pellet (Figure 6c). Sulfuric acid, hydrochloric acid, and hydrofluoric acid each dissolved the tissue, but the result was a dark liquid consisting of highly refractory soluble carbon, and black particulates remained (Figures 6d–6f), indicating incomplete digestion. Peroxide dissolved most of the tissue, but visible white particulates remained after 24 hrs, and these formed a large pellet of presumably non-digestible carbon (Figure 6g). Proteinase K successfully dissolved the tissue (Figure 6h), but without the ATL buffer present, it showed no activity within 24 hrs (not shown), indicating the need for the buffer to achieve activation. On the basis of the visual screening, Solvable, hydroxide, nitric acid, and proteinase K were the most successful at dissolving the rat lung tissue.
Figure 6.
Centirfuged rat lung tissue after treatment with the chemical digestion reagents: (a) Solvable, (b) hydroxide, (c) nitric, (d) sulfuric, (e) hydrochloric, (f) hydrofluoric, (g) peroxide, and (h) proteinase K.
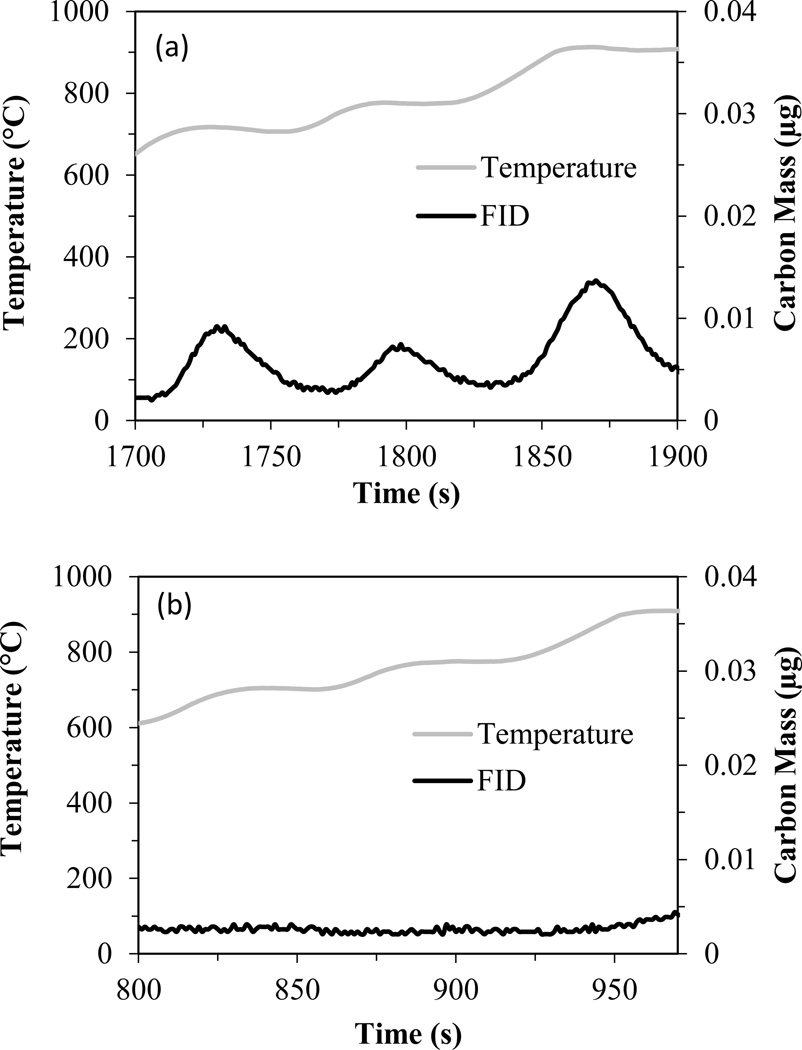
Screening of the different treatment methods indicated that Solvable was the only reagent that had a high recovery efficiency for CNTs and successfully dissolved rat lung tissue. Solvable, which has a low hydroxide concentration (5%), was also ideal because it has a lower risk of corroding PTA instrument parts (e.g., copper tubing, stainless steel, o-rings). For Solvable, the success of the visual experiment was validated by PTA, which was used to analyze the amount of interfering carbon remaining in the treated rat lung tissue pellet. Figure 7a shows the thermogram (oxidation phase only) for the rat lung tissue pellet after treatment with Solvable. Although Solvable was successful at hydrolyzing the tissue and removing most of the interfering carbon, some hydrolysate still remained in the pellet (e.g., Figure 6a), resulting in a small amount (~0.2 µg) of interfering carbon. Although minimal, this interference could be significant if CNT levels approach this value. Proteinase K, which is very effective at cleaving peptide bonds and catalyzing hydrolysis, was used as a follow-up digestion method. Figure 7b shows the thermogram of the rat lung tissue after Solvable treatment followed by proteinase K treatment. The proteinase K successfully eliminated the remaining interfering carbon. This two-step method was selected for extraction of CNTs from rat lung tissue.
Figure 7.
Thermograms for a whole rat lung after treatment with (a) Solvable only and (b) Solvable followed by proteinase K. Data have been cropped to show only the temperatures at which S-CNTs oxidize (i.e., 700–910°C).
Demonstration of Final Extraction Method: Recovery of S-CNTs from Rat Lung Tissue
The final digestion method was used to determine the recovery of S-CNTs from whole rat lungs (dry weight ~0.2 g). First, as a control, the final extraction method was demonstrated on S-CNTs alone. Treatment of 3.6 µg of S-CNTs yielded an average recovery for independent triplicate samples of 3.3 ± 0.09 µg, or 91 ± 2.5% recovery. This indicates minimal loss of S-CNTs as a result of the two-step digestion method with centrifugal washing.
The process was repeated for whole rat lungs spiked with 2.9 ± 0.19 µg S-CNTs. The average recovery for independent triplicate samples was 2.8 ± 0.44 µg or 98 ± 15%. These results show that interfering carbon was removed from the rat lung tissue and the S-CNTs were successfully recovered from the rat lung tissue using the final two-step extraction method developed herein. Based on the similarity of the results from the recovery screening and thermogram evaluation experiments for W-CNTs and S-CNTs treated with Solvable or proteinase K, similar spike recovery results are expected for W-CNTs as well.
SUMMARY AND CONCLUSION
This study considered eight chemicals commonly used to extract metals from complex matrices for application to CNTs. The loss of CNTs was attributed to CNT destruction (e.g., oxidation) and poor pellet formation during CNT separation from the dissolved matrix. Solvable proved to be the most effective at dissolving rat lung tissue while maintaining a high percent recovery of CNTs. This was attributed to its mild nature (i.e., ~2.5% NaOH) and the inclusion of surfactants in the mixture, which presumably helped with pellet formation. Removal of all interfering carbon from rat lung tissue required the development of a two-step digestion method using Solvable and proteinase K. PTA and Raman spectroscopy results showed that treatment of CNTs using Solvable and proteinase K resulted in no apparent structural damage, thus the CNTs remained intact during the extraction procedure. Extraction of 3 µg of S-CNTs from whole rat lungs was efficient with a recovery of 2.8 ± 0.44 µg or 93% ± 15%, indicating the ability of this method to be used in in vivo studies.
The results were applied to rat lung tissue but will have an impact on studies focused on other complex matrices such as composite materials and sediments. If reagents other than Solvable and proteinase K are to be used, both CNT type (i.e., S-CNT or W-CNT) and reagent choice should be considered. For reagents that had issues forming pellets during separation (e.g., nitric acid and hydrofluoric acid), addition of a surfactant mixture may improve recovery by reducing CNT sticking to vials and promoting compact pellets. Given the similarity between CNTs and graphene, these results should be helpful for developing extraction protocols for graphene as well. Using the results presented herein, continued development of extraction methods for CNTs from other complex matrices will be critical for evaluating CNT risk to human health and the environment.
EXPERIMENTAL METHODS
Materials
Two CNTs were used in this study, a thermally weak CNT with a high defect density (W-CNT, Cheaptubes, Inc., MWCNT, 20–30 nm diameter, 10–30 µm length, as reported by manufacturer) and a thermally strong CNT with a relatively low defect density (S-CNT, Mitsui & Co., MWCNT, 40–70 nm diameter, 10–40 µm length, as reported by manufacturer and verified by us). The W-CNTs and the S-CNTs were dispersed in a medium that has been used previously in rat lung CNT exposure studies.23 A functionalized version of the W-CNT was provided by Dr. Som Mitra.24 The dispersion media consisted of bovine serum albumin (BSA, Sigma Aldrich A-2058, >97%), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, Sigma Aldrich P-0763, >99%), and sodium chloride (NaCl, Sigma Aldrich S7653, >99.5%). Chemicals used for digestion included nitric acid (HNO3, EMD NX0409-2, 67–70% (15.8 M)), sulfuric acid (H2SO4, JT Baker 6902-05, 98% (18.4 M)), hydrogen peroxide (H2O2, JT Baker 5155-01, 30% (9.8 M)), hydrochloric acid (HCl, EMD HX0603-3, 32–36% (11 M)), hydrofluoric acid (HF, JT Baker 6904-05, 40% (22.6 M)), ammonium hydroxide (NH4OH, Sigma Aldrich 320145, 28–30% (14.8 M)), Solvable (Perkin Elmer, 2.5% NaOH, 2.5–10% N,N-dimethyldodecylamine N-oxide, 2.5–10% polyethylene glycol trimethylnonyl ether), and proteinase K (20 g/L)/ATL buffer (QIAGEN 19131/19076). The ATL buffer composition is mostly proprietary; the known components include sodium dodecyl sulfate (2.5–10%) and edetic acid. The proteinase K mixture was prepared by mixing 20 µL of proteinase K, 180 µL of ATL buffer, and 800 µL of water (total volume = 1 mL). Rat lungs were obtained from male (Rockland, Inc.) and female Sprague-Dawley rats (250–400 g body weight).
Preparation of CNT Suspensions
Dispersion medium was prepared as described previously.23 Briefly, 10 mL of dispersion medium was prepared using 3.99 mL 0.9% NaCl, 6 mL of BSA (1 mg/mL in 0.9% NaCl), and 0.01 mL of DPPC (10 mg/mL in ethanol, made fresh each time). CNTs were then added to the dispersion medium to obtain a concentration of 0.5 mg CNT/mL. The dispersion was stirred briefly and then probe sonicated (Sonics VibraCell VCX750, 3 mm microtip) in an ice bath for 10 minutes at 50% duty cycle (10 s on, 10 s off), as described previously for preparing nanoparticle suspensions for in vivo and in vitro studies.25
Programmed Thermal Analysis
PTA was used to determine CNT recovery, determine changes in CNT thermal stability after treatment, and quantify rat lung tissue background carbon after treatment; PTA operation is described in detail elsewhere.13 Briefly, samples were heated using a CNT-specific temperature ramp program in 100% He (inert conditions) and then in 90%He/10% O2 (oxidizing conditions; see Figure SI-1). The carbon that evolves during analysis is converted to methane and then detected using flame ionization (FID). This FID signal is calibrated with internal and external standards and then used to calculate the mass of carbon evolved. The initial inert heating process is used to remove any organic carbon remaining after digestion, and the oxidizing heating program is used to develop the CNT thermogram. The maximum temperature under inert conditions was set at 675°C to avoid loss of W-CNTs, as found previously13 The thermogram and the time required to reach 50% CNT mass loss was used to determine the effect of the treatment methods on the thermal stability of the CNTs.
Raman Spectroscopy
Raman spectroscopy was used to determine the changes in the CNT structure (i.e., defects) due to the various treatment methods. Raman spectroscopy was performed on a custom-built instrument in 180° geometry. The sample was excited using a 532-nm laser with 100-mW maximum power, which was controlled using neutral density filters. The data were collected using an Acton 300i spectrograph and a back-thinned Princeton Instruments liquid nitrogen–cooled CCD detector with a spatial resolution <1 µm and spectral resolution of ~1 cm−1. Between 1300 and 1600 cm−1, there are two distinct peaks for CNTs, called the D-band and the G-band. The D-band is present because of defects or disorder present within the CNT sample and increases in intensity with increasing disorder. The G-band is the graphitic band, and a higher, narrower peak indicates a more ordered CNT. The ratio of the D-band and G-band intensities (ID/IG) is often used to determine the defectiveness of CNTs, and this ratio may be used to estimate the thermal stability of CNTs.26–31 For this study, the average ID/IG ratio was calculated from four measurements taken at different points for each sample.
Procedure for Screening Chemical Digestion Reagents for CNTs and Rat Lung Tissue
Reagents examined included nitric acid (“nitric”), sulfuric acid (“sulfuric”), hydrofluoric acid (“hydrofluoric”), hydrochloric acid (“hydrochloric”), hydrogen peroxide (“peroxide”), Solvable, sodium hydroxide (“hydroxide”), and proteinase K. CNTs and rat lung tissue were screened separately. Briefly, CNT or rat lung tissue samples were placed in a 2 mL plastic centrifuge tube, and the appropriate digestion reagent was added. For CNTs, approximately 25 µg were added to 1 mL of digestion reagent. For rat lung tissue, samples were dried in air at 110°C, ground to a powder using an agate mortar and pestle, and then 10 mg were added to 1 mL of digestion reagent. The samples were heated at 60°C and mixed (30 s on at 700 RPM, 5 min off) for 24 hrs. After treatment, samples were washed with water using centrifugal separation at 25,000 × g for 15 minutes. Acidic and alkaline samples were neutralized with 1 M NaOH and 1 M HNO3, respectively, during the initial washing steps (~30 µL added). After washing, sample pellets consisting of CNTs and undigested rat lung tissue (for inefficient reagents) were loaded onto pre-fired quartz-fiber filters (QFFs, Pall Tissuquartz Filters, 2500 QAT-UP), wrapped in pre-fired aluminum foil, dried in air for 1 hr to prevent sticking to the foil, and then dried in a furnace at 90°C. Filtration directly onto the QFF was examined as an alternative separation method to centrifugation, but some CNTs passed through the filter. No replicates were performed for screening experiments.
Extraction of CNTs from Rat Lung Tissue
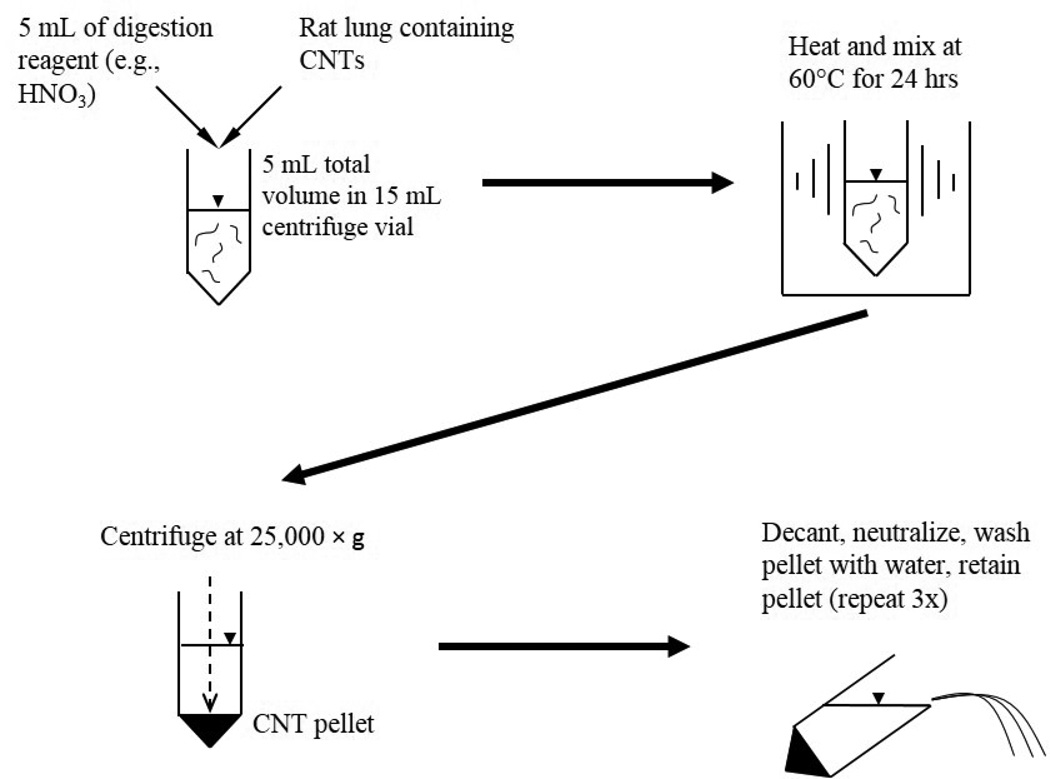
The final extraction method was demonstrated using whole rat lungs and CNTs in independent triplicate samples. CNTs in dispersion medium were spiked directly onto fresh lung tissue. The sample was then dried in air at ~110°C, and the extraction was performed in the same vessel without grinding to avoid potential CNT losses. Dried lung tissue was used so the CNT mass could be normalized to the lung mass for equal comparison between samples. Similar results were obtained using wet lung tissue, so the drying step could be eliminated if this was the desired application. For whole lungs, the extraction procedure was similar to that described in the screening experiments, but a 15-mL round-bottom centrifuge vial and 5 mL of the appropriate digestion reagent were used. The extraction protocol is outline in Figure 1. As a control, CNTs in dispersion medium were injected directly onto a QFF and quantified by PTA; this result was used to define the percent recovery of CNTs. The target CNT dose for these experiments was 3 µg, which is in the appropriate range (1–10 µg) as predicted by a particle deposition model simulating inhalation of CNTs for 4 hrs at the occupational exposure limit of 2 mg/m3 for elemental carbon.32
Figure 1.
Chemical digestion procedure for rat lung tissue and CNTs.
Supplementary Material
Acknowledgements
This work was supported by grants from NIEHS (RC2 ES018801 and RC2 ES018741) and the Semiconductor Research Corporation (ERC-425.040). We greatly appreciate Dr. Matt Fraser for providing access to the PTA instrument. Instruments used for material characterization were provided by the ASU LeRoy Eyring Center for Solid State Science. We also thank Dr. Som Mitra, New Jersey Institute of Technology, for providing the functionalized W-CNTs.
Footnotes
Supporting Information Available. Sample PTA temperature program, photographs detailing the separation method, and thermograms of CNTs after treatment. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Endo M, Strano MS, Ajayan PM. Potential Applications of Carbon Nanotubes. Carbon Nanotubes. 2008;111:13–61. [Google Scholar]
- 2.Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to Carbon Nanotube Material: Aerosol Release During the Handling of Unrefined Single-Walled Carbon Nanotube Material. J. Toxicol.Environ. Health, Part A. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- 3.Aschberger K, Johnston HJ, Stone V, Aitken RJ, Hankin SM, Peters SAK, Tran CL, Christensen FM. Review of Carbon Nanotubes Toxicity and Exposure-Appraisal of Human Health Risk Assessment Based on Open Literature. Crit. Revi. Toxicol. 2010;40:759–790. doi: 10.3109/10408444.2010.506638. [DOI] [PubMed] [Google Scholar]
- 4.Han JH, Lee EJ, Lee JH, So KP, Lee YH, Bae GN, Lee SB, Ji JH, Cho MH, Yu IJ. Monitoring Multiwalled Carbon Nanotube Exposure in Carbon Nanotube Research Facility. Inhalation Toxicol. 2008;20:741–749. doi: 10.1080/08958370801942238. [DOI] [PubMed] [Google Scholar]
- 5.Kuhlbusch TAJ, Asbach C, Fissan H, Gohler D, Stintz M. Nanoparticle Exposure at Nanotechnology Workplaces: A Review. Part. Fibre Toxicol. 2011;8:18. doi: 10.1186/1743-8977-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, Alexander A. Carbon Nanotubes: A Review of Their Properties in Relation to Pulmonary Toxicology and Workplace Safety. Toxicol. Sci. 2006;92:5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- 7.Ma-Hock L, Treumann S, Strauss V, Brill S, Luizi F, Mertler M, Wiench K, Gamer AO, van Ravenzwaay B, Landsiedel R. Inhalation Toxicity of Multiwall Carbon Nanotubes in Rats Exposed for 3 Months. Toxicol. Sci. 2009;112:468–481. doi: 10.1093/toxsci/kfp146. [DOI] [PubMed] [Google Scholar]
- 8.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, Stone V, Brown S, MacNee W, Donaldson K. Carbon Nanotubes Introduced into the Abdominal Cavity of Mice Show Asbestos-Like Pathogenicity in a Pilot Study. Nat. Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 9.Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, Hubbs AF, Mercer RR, Keohavong P, Sussman N, et al. Inhalation Vs. Aspiration of Single-walled Carbon Nanotubes in C57bl/6 Mice: Inflammation, Fibrosis, Oxidative Stress, and Mutagenesis. Am. J. of Physiol.-Lung C. 2008;295:L552–L565. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauluhn J. Subchronic 13-Week Inhalation Exposure of Rats to Multiwalled Carbon Nanotubes: Toxic Effects Are Determined by Density of Agglomerate Structures, Not Fibrillar Structures. Toxicol. Sci. 2010;113:226–242. doi: 10.1093/toxsci/kfp247. [DOI] [PubMed] [Google Scholar]
- 11.Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, et al. Inhaled Carbon Nanotubes Reach the Subpleural Tissue in Mice. Nat. Nanotechnol. 2009;4:747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen EJ, Henry TB. Methodological Considerations for Testing the Ecotoxicity of Carbon Nanotubes and Fullerenes: Review. Environ. Toxicol. Chem. 2012;31:60–72. doi: 10.1002/etc.710. [DOI] [PubMed] [Google Scholar]
- 13.Doudrick K, Herckes P, Westerhoff P. Detection of Carbon Nanotubes in Environmental Matrices Using Programmed Thermal Analysis. Environ. Sci. and Technol. 2012;46:12246–12253. doi: 10.1021/es300804f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NIOSH, Elemental Carbon (Diesel Particulate): Method 5040. NIOSH Manual of Analytical Methods. 4th Edition ed. National Institute for Occupational Safety and Health; 1998. [Google Scholar]
- 15.Osswald S, Havel M, Gogotsi Y. Monitoring Oxidation of Multiwalled Carbon Nanotubes by Raman Spectroscopy. J. Raman Spectrosc. 2007;38:728–736. [Google Scholar]
- 16.USEPA, SW-846 EPA Method 3050B, Acid Digestion of Sediments, Sludges and Soils. Test Methods for Evaluating Solid Waste. 3rd ed. Washington DC: US Environmental Protection Agency; 1995. [Google Scholar]
- 17.USEPA. Washington DC: US Environmental Protection Agency; 1996. SW-846 EPA Method 3052, Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices. [Google Scholar]
- 18.Griffis LC, Wolff RK, Henderson RF, Griffith WC, Mokler BV, McClellan RO. Clearance of Diesel Soot Particles from Rat Lung After a Subchronic Diesel Exhaust Exposure. Fundam. Appl. Toxicol. 1983;3:99–103. doi: 10.1016/s0272-0590(83)80063-3. [DOI] [PubMed] [Google Scholar]
- 19.Searl A, Cullen RT. An Enzymatic Tissue Digestion Method for Fibre Biopersistence Studies. Ann. Occup. Hyg. 1997;41:721–727. doi: 10.1016/s0003-4878(97)00023-9. [DOI] [PubMed] [Google Scholar]
- 20.Peng Y, Liu HW. Effects of Oxidation by Hydrogen Peroxide on the Structures of Multiwalled Carbon Nanotubes. Ind. Eng. Chem. Res. 2006;45:6483–6488. [Google Scholar]
- 21.Rosca ID, Watari F, Uo M, Akaska T. Oxidation of Multiwalled Carbon Nanotubes by Nitric Acid. Carbon. 2005;43:3124–3131. [Google Scholar]
- 22.Tchoul MN, Ford WT, Lolli G, Resasco DE, Arepalli S. Effect of Mild Nitric Acid Oxidation on Dispersability, Size, and Structure of Single-walled Carbon Nanotubes. Chem. Mater. 2007;19:5765–5772. [Google Scholar]
- 23.Porter D, Sriram K, Wolfarth M, Jefferson A, Schwegler-Berry D, Andrew M, Castranova V. A Biocompatible Medium for Nanoparticle Dispersion. Nanotoxicol. 2008;2:144–154. [Google Scholar]
- 24.Xia T, Hamilton RF, Jr, Bonner JC, Crandall ED, Elder A, Fazlollahi F, Girtsman TA, Kim K, Mitra S, Ntim SA, et al. Interlaboratory Evaluation of In Vitro Cytotoxicity and Inflammatory Responses to Engineered Nanomaterials: The NIEHS Nano GO Consortium. Environ. Health Perspect. 2013;121:683–690. doi: 10.1289/ehp.1306561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonner JC, Silva RM, Taylor AJ, Brown JM, Hilderbrand SC, Castranova V, Porter D, Elder A, Oberdörster G, Harkema JR, et al. Interlaboratory Evaluation of Rodent Pulmonary Responses to Engineered Nanomaterials: the NIEHS Nano GO Consortium. Environ. Health Perspect. 2013;121:676–682. doi: 10.1289/ehp.1205693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behler K, Osswald S, Ye H, Dimovski S, Gogotsi Y. Effect of Thermal Treatment on the Structure of Multi-Walled Carbon Nanotubes. J. Nanopart. Res. 2006;8:615–625. [Google Scholar]
- 27.Bhalerao GM, Sinha AK, Sathe V. Defect-dependent Annealing Behavior of Multi-Walled Carbon Nanotubes. Physica E Low Dimens Syst Nanostruct. 2008;41:54–59. [Google Scholar]
- 28.Chen J, Shan JY, Tsukada T, Munekane F, Kuno A, Matsuo M, Hayashi T, Kim YA, Endo M. The Structural Evolution of Thin Multi-Walled Carbon Nanotubes During Isothermal Annealing. Carbon. 2007;45:274–280. [Google Scholar]
- 29.Kim YA, Hayashi T, Osawa K, Dresselhaus MS, Endo M. Annealing Effect on Disordered Multi-Wall Carbon Nanotubes. Chem. Phys. Lett. 2003;380:319–324. [Google Scholar]
- 30.Singh DK, Iyer PK, Giri PK. Diameter Dependence of Oxidative Stability in Multiwalled Carbon Nanotubes: Role of Defects and Effect of Vacuum Annealing. J. Appl. Phys. 2010;108:10. [Google Scholar]
- 31.Tran MQ, Tridech C, Alfrey A, Bismarck A, Shaffer MSP. Thermal Oxidative Cutting of Multi-Walled Carbon Nanotubes. Carbon. 2007;45:2341–2350. [Google Scholar]
- 32.Asgharian B, Anjilvel S. A Multiple-Path Model of Fiber Deposition in the Rat Lung. Toxicol. Sci. 1998;44:80–86. doi: 10.1006/toxs.1998.2476. [DOI] [PubMed] [Google Scholar]
- 33.Dresselhaus MS, Dresselhaus G, Saito R, Jorio A. Raman Spectroscopy of Carbon Nanotubes. Phys. Rep.-Rev. Sect. Phys. Lett. 2005;409:47–99. [Google Scholar]
- 34.Schonfelder R, Aviles F, Bachmatiuk A, Cauich-Rodriguez JV, Knupfer M, Buchner B, Rummeli MH. On the Merits of Raman Spectroscopy and Thermogravimetric Analysis to Asses Carbon Nanotube Structural Modifications. Appl. Phys. A:Mater. Sci. Process. 2012;106:843–852. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.