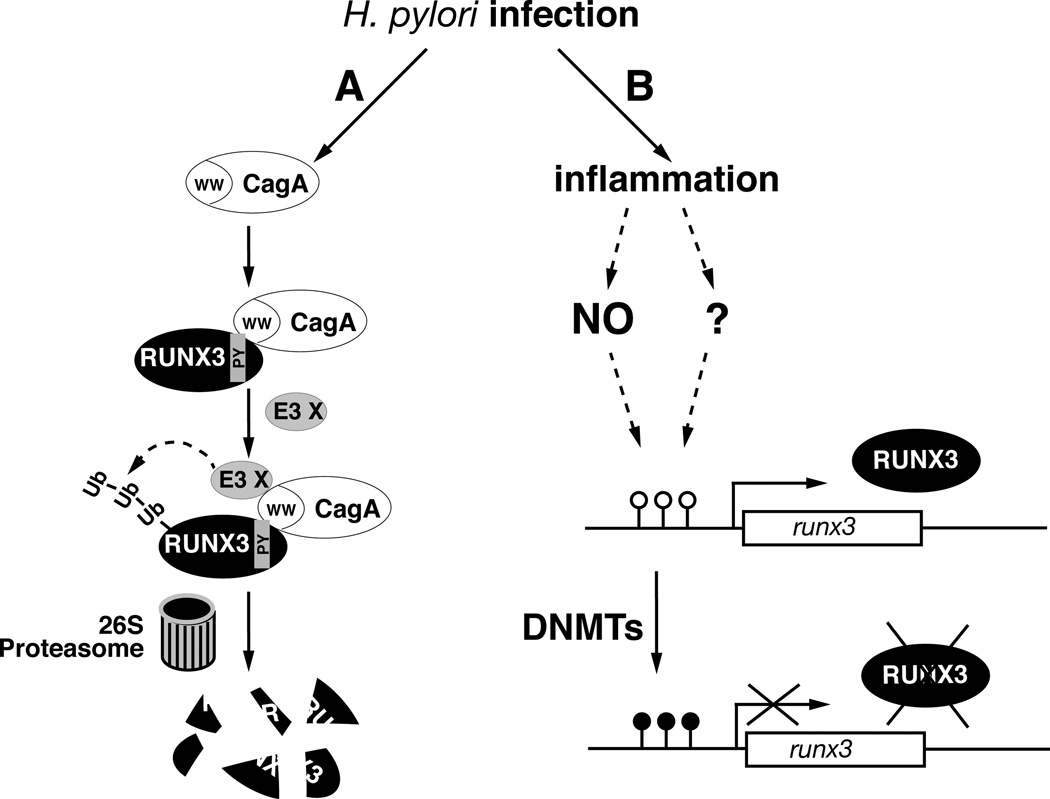
Fig. 1.
Inactivation of tumor suppressor RUNX3 by H. pylori infection. (A) CagA-dependent inactivation of RUNX3 by proteolytic degradation. CagA is injected into host epithelial cells after H. pylori infection. Within the cells, it interacts with the Py motif of RUNX3 via its WW domain. Binding of CagA to RUNX3 recruits an unidentified E3 ubiquitin ligase and triggers the proteasome-mediated degradation of RUNX3. This rapid inactivation of RUNX3 during the early stage of infection might promote the proliferation and survival of gastric epithelial cells and the growth of the H. pylori. (B) Inactivation of RUNX3 by hypermethylation of the runx3 promoter. H. pylori infection induces the hypermethylation of the runx3 promoter. This epigenetic-level inactivation of RUNX3 is associated with H. pylori infection-induced inflammation, which results in abnormal activity of DNMTs and the production of nitric oxide (NO). Solid arrow: known interaction or inactivation; arrow with question mark: proposed interaction; dashed arrow: activation through multiple steps. ○ =CpG, ●=mCpG.