Abstract
Pimenta pseudocaryophyllus is a Brazilian native plant that presents high concentrations of flavonoids and other polyphenolic compounds. Herein, we evaluated: (1) the chemical properties of P. pseudocaryophyllus ethanolic extract (PPE), (2) the in vitro antioxidant activity (AA) of PPE and of two different topical formulations (F1 and F2) containing PPE, (3) physico-chemical and functional stability, (4) in vitro release of PPE, and (5) in vivo capacity of formulations to prevent UV-B irradiation-induced skin damage. Results show that the polyphenol and flavonoid contents in PPE were 199.33 and 28.32 mg/g, respectively, and HPLC results show the presence of eugenol, tannic acid, and rutin. Evaluation of the in vitro AA of PPE demonstrated a dose-dependent effect and an IC50 of 4.75 μg/mL in 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 3.0 μg/mL in 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays. The ferric-reducing antioxidant power (FRAP assay) was 0.046 μmol/L trolox equivalent/μg/mL of extract. Among the AA, only the capacity to scavenge DPPH radical of PPE was maintained in F1 and F2. In addition, both formulations satisfactorily released the extract. The evaluation of the functional stability of F1 and F2 did not demonstrate loss of activity by storage at room temperature and at 4°C/6 months. In irradiated mice, treatment with F1 and F2 added with PPE significantly increased the capacity to scavenge ABTS radical and the FRAP of skin compared to vehicle-treated mice. In conclusion, the present results suggest that formulations containing PPE may be a topical source of antioxidant compounds to decrease oxidative damages of the skin.
KEY WORDS: antioxidant, oxidative stress, Pimenta pseudocaryophyllus, topical formulations, UV-B irradiation
INTRODUCTION
Among all the cellular sources of reactive oxygen species (ROS), ultraviolet (UV) radiation plays the most prominent role in the induction of cutaneous oxidative stress. Acute exposure to UV irradiation causes sunburn, DNA damage, and connective tissue degradation. Accumulated damage resulting from chronic sun exposure can cause skin cancer and premature skin aging (photoaging) (1–3).
Epidemiological studies indicate that the use of sunscreens and sun blockers are not completely effective in preventing UV irradiation-induced skin cancer (4–6), thus, new targeted chemopreventive approaches need to be identified (7). Considering the deleterious effects of ROS in the skin, many studies have focused on the establishment and evaluation of antioxidants to enrich the endogenous cutaneous protection system, and to prevent and/or treat UV irradiation-induced skin damage. In this context, much attention has been paid to antioxidants from natural sources, especially flavonoids and other phenolic compounds (8–10).
Pimenta pseudocaryophyllus is present in the Atlantic forest and Brazilian Cerrado. Commonly, teas prepared with its leaves are used as tranquilizers, digestive regulators, and for the relief of cold symptoms (11). Despite being the only species of the gender native to Brazil (12), there are still few studies with this plant. The leaves of P. pseudocaryophyllus present high concentrations of polyphenolic compounds such as tannins and flavonoids (11,12), which suggests that it might have the ability to act as an antioxidant. Corroborating, it was reported that the antioxidant activity (AA) could be used to evaluate the functional activity of topical functionalized formulations (13,14) and the release of antioxidant components from these formulations (15,16).
Thus, the evaluation of topical formulations containing plant extracts by in vitro AA and in vivo efficacy is a crucial issue in the study of new pharmaceutical products for skin protection against UV radiation-induced damage. Furthermore, there is no evidence of in vivo use of topical formulation containing PPE to prevent oxidative damages. In this context, the present study was designed to evaluate the chemical composition of PPE, the AA of PPE alone, and in different topical formulations, in addition to the in vitro release of antioxidant compounds. Furthermore, physico-chemical and functional stabilities were also assessed. Finally, the in vivo protection of the formulations against oxidative stress caused by UV-B irradiation in hairless mice was evaluated.
MATERIAL AND METHODS
Chemicals
Quercetin dihydrate 99% (C15H10O7·2H2O, Mw = 338.26) and quercetin-3-O-rutinoside (rutin) were purchased from Acros Organics (New Jersey, USA). Folin-Ciocalteau was obtained from Fluka Chemical Co. (Buchs, Switzerland) and propylene glycol from Chemco LTDA. 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,4,6-tris(2-pyridyl-s-triazine (TPTZ), tannic acid, and gallic acid were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Nitrocellulose membrane 0.45 μm, 47 mm, model HAWP04700, white HAWP was obtained from Millipore (Sao Paulo, SP, Brazil). Eugenol was obtained from Vetec (Rio de Janeiro, Brazil). Raw materials for formulations were obtained from Galena (Campinas, SP, Brazil). All other reagents used were of pharmaceutical grade.
Plant Material and Extract Preparation
The leaves of P. pseudocaryophyllus were collected in December 2007 at São Jerônimo da Serra (Paraná, Brazil). The plant specimens were identified by A.O.S. Vieira, Departamento de Biologia Animal e Vegetal (Centro de Ciências da Saúde) and a voucher specimen was deposited at the “Herbarium of Universidade Estadual de Londrina (FUEL)” under code no. 43025. The plant material was dried at 40°C and coarsely powdered in industrial blender. The ethanolic extract (1:10) was obtained by exhaustive maceration at room temperature (RT; 25°C) for 12 days. The extract was filtered and concentrated under vacuum.
Chemical Characteristics of PPE
Total Flavonoids and Polyphenol Contents of PPE
Total polyphenol content in PPE was determined by the Folin–Ciocalteau colorimetric method (13,17); 0.5 mL of PPE solution was mixed with 0.5 mL of the Folin–Ciocalteau reagent and 0.5 mL of 10% Na2CO3, and after 1 h of incubation at RT the absorbance was measured at 760 nm. Total polyphenol content was expressed as milligrams per gram (gallic acid equivalents). Total flavonoid content was determined using the aluminium chloride colorimetric method (18). To 0.5 mL of PPE solution, 0.5 mL of 2% AlCl3 ethanolic solution was added. After 1 h at RT, the absorbance was measured at 420 nm. Total flavonoid contents were calculated as quercetin (mg/g) from an analytical curve.
High-Performance Liquid Chromatography Analysis
The extract was analyzed by high-performance liquid chromatography (HPLC; Shimadzu) equipped with a photodiode array detector (SPD-M10Avp), multisolvent delivery system (LC-10Avp), oven control system (CTO-10ASvp), and controlled software Class VP 6.14 software. Chromatography was performed on an analytical reverse-phase column Spherisob® (C-18 ODS) (250 × 4.6 mm i.d.; particule size 5 μm; Waters). The HPLC-grade solvents were supplied by Panreac®, and water was purified using Milli-Q-plus filter systems (Millipore). For HPLC runs, a gradient of acidified H2O (2% formic acid; solvent A) and acetonitrile (2% formic acid; solvent B) was used at a flow rate of 1 mL/min, and the volume injected was 20 μL (0 min, 0% B; 5 min, 0% B; 20 min, 2.5% B; 30 min, 5% B; 50 min, 15% B; 60 min, 25% B; 65 min, 30% B; 70 min, 45% B; 75 min, 50% B; 80 min, 70% B; 85 min, 90% B; 90 min, 100% B; 95 min, 100% B; 110 min, 0% B).
UV detection was performed at 200–400 nm (scan) and then set to 280 and 340 nm. UV spectra were recorded for each main peak in the chromatograms. The following compounds were used as references (external standard): quercetin-3-O-rutinoside (rutin, Sigma), tannic acid, and eugenol (19).
Determination of In Vitro Antioxidant Efficacy of PPE
Hydrogen-Donating Ability by DPPH Assay
To measure the ability of PPE to scavenge DPPH radical, 20 μL of PPE sample (1 to 20 μg/mL in medium reaction, based on PPE total solids content of 25 mg/mL) added to the reaction mixture containing 1 mL of 0.1 M acetate buffer (pH 5.5), 1 mL of ethanol, and 0.5 mL of ethanolic solution of DPPH 250 μM. The absorbance was measured at 517 nm after 15 min of incubation at RT in a Thermo Scientific Evolution® 60 spectrophotometer. The positive control was prepared in the absence of PPE, and it indicates the maximum odd electrons of DPPH, which was considered 100% of free radicals in the solution to calculate the hydrogen-donating ability (%) of PPE. The blank was prepared from the reaction mixture without DPPH solution (20,21). The ability of scavenging DPPH was calculated by the following equation
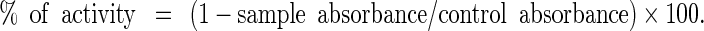 |
1 |
Scavenging Ability of PPE Using ABTS Method
The ability to scavenge the ABTS free radical is measured by an absorbance decrease due to suppression of the colored radical (22). The method was carried out according to Sánchez-Gonzalez et al. (23), with some modifications. Radical cation ABTS was obtained after the reaction of 7 mM ABTS stock solution with 2.45 mM potassium persulfate at room temperature in the dark for 16 h before use. This solution was diluted in phosphate buffer (pH 7.4, 0.1 M) until it reached an absorbance of 0.7 at 730 nm. Fifty microliters of PPE samples were added to 4 mL of the diluted ABTS solution. The concentrations of PPE obtained in the reaction medium were 0.625–15.625 μg/mL (based on PPE total solids content of 25 mg/mL). The absorbance was measured at 730 nm after 6 min of incubation at RT in a Thermo Scientific Evolution® 60 spectrophotometer. The positive control was prepared in the absence of PPE and was considered 100% of free radicals in the solution to calculate the scavenging ability of PPE. The ability of scavenging ABTS was calculated using Eq. 1.
Evaluation of the Ferric-Reducing Antioxidant Power of PPE
The ferric reduction antioxidant power of PPE was evaluated according to Sánchez-Gonzalez et al. (2005) (23), with some modifications. Ferric-reducing antioxidant power (FRAP) reagent was prepared with 2.5 mL of TPTZ solution (10 mM) in HCl (40 mM), 2.5 mL of 20mM FeCl3 (6H2O) solution, and 25 mL of acetate buffer (pH 3.6, 0.3 mM). The solution was incubated at 37°C for 30 min. For the assay, 900 μL of FRAP reagent were added to 90 μL of water and 10 μL of trolox standard or 10 μL of PPE (1.56 μg/mL in the reaction medium). After incubation at 37°C for 30 min, the measurement was performed in a spectrophotometer (Thermo Scientific Evolution® 60) at 595 nm. An analytical curve with different concentrations of trolox (4.0–20.0 μM) was used for subsequent calculation of results in micromoles per liter of trolox equivalent per micrograms per milliliter of extract. A positive control was performed with ethanol.
Formulations
Formulations were developed varying the content of lipidic. Self-emulsifying wax Polawax® (cetostearyl alcohol + polyoxyethylene derived of a fatty acid ester of sorbitan 20E) was used in both formulations, although in major and minor proportions in formulation 1 (F1) and formulation 2 (F2), respectively. Into F2, anionic hydrophilic colloid (carboxypolymethylene, Carbopol®) was also added as stabilizing agent. Caprylic/capric triglycerides was used as emollient, and propylene glycol as moisturizer. A mixture of parabens was used as preservative and deionized water was used for the preparation of all formulations (Table I). PPE was incorporated (5%) into the formulations at RT. Control formulations did not contain the extract.
Table I.
Percent Composition (Weight/Weight) of F1 and F2
| Components | F1 | F2 |
|---|---|---|
| Polawax® | 10.0 | 2.0 |
| Caprylic/capric triglyceride | 5.0 | 5.0 |
| Carbopol® 940 | – | 0.18 |
| Propylene glycol | 5.0 | 5.0 |
| Triethanolamine | – | 0.2 |
| Solution of methyl (10%) and propylparaben (2%) | 1.0 | 1.0 |
| Deionized water | 79.0 | 86.62 |
Evaluation of Physico-chemical Characteristics of Formulations
With the aim of evaluating the physico-chemical characteristics, the following tests were performed: visual evaluation (color, consistence, and phase separation) (24); pH measurement in triplicate (10% dilution in deionized water); evaluation of phase separation in triplicate (2 g of formulation submitted to centrifugation at 145 × g for 30 min) (13,25).
Evaluation of the Antioxidant Activity of F1 and F2 Containing PPE
In order to evaluate the AA of PPE after its incorporation to F1 and F2, DPPH, ABTS, and FRAP methods were performed as described in Section “Determination of In Vitro Antioxidant Efficacy of PPE”. Formulations containing PPE were diluted in ethanol to obtain the same concentration used for the analysis of PPE in the reaction medium: 5.0, 3.125, and 2.5 μg/mL for DPPH, ABTS, and FRAP, respectively. The following controls were included in the test: (1) one positive control was prepared in the absence of sample, and (2) another by adding the formulations without PPE.
Physico-chemical and Functional Stability of Formulations Containing PPE
Physico-chemical and functional stability were evaluated by submitting the formulations to storage at 4°C, RT (25°C), and 40 ± 2°C/75 ± 5% of relative humidity (RH) for 6 months (24). The extract was also stored in the same storage conditions for evaluation of its functional stability. At predetermined time intervals (0, 30, 60, 90, and 180 days), aliquots were collected and analyzed. The physico-chemical stability of the formulations was determined by the tests described in Section “Evaluation of Physico-chemical Characteristics of Formulations” and functional stability of PPE, F1, and F2 was measured by DPPH method as described in Section “Hydrogen-Donating Ability by DPPH Assay”. One positive control for each storage condition was added. The positive control was prepared in the absence of PPE and another added with formulations without PPE which indicates the maximum odd electrons of DPPH.
In Vitro Release Studies
PPE release rates from the different formulations were measured through 0.45 μm nitrocellulose membranes using modified Franz diffusion cells with a diffusional area of 1.77 cm2 (20). The membrane was sandwiched between the upper donor compartment and the lower receptor compartment; 1 g of F1 or F2 containing 5% of PPE was placed on the membrane surface in the donor compartment while the receptor compartment was filled with 16 mL of receptor medium (0.1 M phosphate buffer (pH 7.4) with 10% of ethanol), which was in contact with the membrane. During the experiments, the receptor solution was continuously stirred at 100 rpm and kept at 37 ± 1°C. At designated time points (3, 6, 9, and 12 h) the receptor medium was removed and the release of PPE antioxidant compounds on F1 and F2 was analyzed by DPPH method (see Section Hydrogen-Donating Ability by DPPH Assay). Results are expressed as percentage of PPE released by the formulations. All measurements were performed in duplicate and formulations without PPE were used as control.
In Vivo Studies
Animals
Sex-matched hairless mice (HRS/J), weighing 20–30 g, were housed in a temperature-controlled room, with access to water and food ad libitum until use. All experiments were conducted in accordance with National Institutes of Health guidelines for the welfare of experimental animals and with the approval of the Ethics Committee of State University of Londrina (Protocol number 34994/209).
Formulation Administration
Hairless mice were randomly designated to different groups (n = 5) and topically treated on the dorsal surface with 0.5 g of F1 or F2 with 5% of PPE or 0.5 g of the respective control formulation without PPE. Formulations were administrated 1 h before, 5 min before, and right after the irradiation. Untreated control groups irradiated and non-irradiated were included in the experiments. The results are representative of two separate experiments.
Irradiation
The UV-B source of irradiation consisted of a Philips TL40W/12 RS lamp (Medical-Holand) emitting a continuous spectrum between 270 and 400 nm with a peak emission at 313 nm. The lamp was mounted 20 cm above the table where the mice were placed on, resulting in an irradiation of 0.384 mW/cm2 as measured by an IL 1700 radiometer (Newburyport, MA, USA) with sensor for UV (SED005) and UV-B (SED240). The dose of UV-B used was 4.14 J/cm2 (26,27). Mice were euthanized by ketamine/xylazine overdose 12 h after the UV-B exposure, and the full thickness of the dorsal skins were removed. ABTS and FRAP tests were performed right after sample collection.
Sample Preparation
Skin samples of the animals were collected in Eppendorf tubes containing 500 μL of 1.15% KCl solution. After, samples were homogenized with Tissue-Tearor (Biospec®). A centrifugation at 1,000 × g for 10 min at 4°C was performed and the supernatant was used for the assays.
Scavenging Ability of Skin Using ABTS Method
The ability of ABTS radical scavenging was performed as described in Section “Scavenging Ability of PPE Using ABTS Method”, in which 40 μL of the supernatant was added to 1 mL of the diluted ABTS solution. An analytical curve with different concentrations of trolox (1–25 μM) was used for subsequent calculation of results in micromoles per liter of trolox equivalent per milligram of skin (28). The assay was performed in duplicate.
Evaluation of the Ferric-Reducing Antioxidant Power of Skin
The FRAP was performed as described in Section “Evaluation of the Ferric Reducing Antioxidant Power (FRAP) of PPE”, in which 30 μL of supernatant were added to 1 mL of FRAP reagent. An analytical curve with different concentrations of trolox (0.5–20 μM) was used for subsequent calculation of results in micromoles per liter of trolox equivalent per milligram of skin (28). The assay was performed in duplicate.
Statistical Analysis
The concentration of PPE that caused 50% of DPPH and ABTS scavenging was considered the IC50, which was determined using GraphPad Prism® software, version 6.0 using hyperbolic curve (one site binding and two site binding hyperbole). Data were statistically analyzed by one-way ANOVA followed by Bonferroni's t test for antioxidant activity of the formulations containing PPE, in vitro release and in vivo tests. Results were expressed as means ± SEM (standard error mean) and considered significantly different when P < 0.05.
RESULTS
Chemical Characteristics of PPE
Results show that PPE presents 199.33 ± 3.79 and 28.32 ± 1.46 mg/g of polyphenols and flavonoids, respectively. Eugenol, rutin, and tannic acid as reference compounds were identified in the ethanolic extract (Fig. 1). The individual flavonoids and phenolic compounds were identified by HPLC-PDA and comparison of UV data (λmax) of reported value.
Fig. 1.

Identification of phenolic components of P. pseudocaryophyllus ethanolic extract using high-performance liquid chromatography
In Vitro Antioxidant Activity of PPE
The hydrogen-donating ability of PPE was evaluated by the use of the stable radical DPPH as presented in Fig. 2a. The maximum antioxidant activity was 88.17% using the concentration of 20 μg/mL of PPE and the IC50 was 4.75 μg/mL. Regarding ABTS method, results showed that PPE exhibited effective and concentration-dependent scavenging activity (Fig. 2b). The IC50 was 3.0 μg/mL and the maximum activity (7.82 μg/mL) was approximately 98.89%, in which a plateau was observed. In FRAP assay, PPE-reducing power was 0.046 μmol/L trolox equivalent/μg/mL of extract.
Fig. 2.

(a) H-donor ability of PPE using stable radical DPPH (concentrations of PPE on the reaction medium, 1–20 μg/mL). (b) Scavenging ability of PPE using ABTS method (concentrations of PPE on the reaction medium, 0.625–15.625 μg/mL). Results are represented by means ± SEM of three separated experiments
Formulations
Evaluation of Antioxidant Activity of the Formulations Containing PPE
In order to verify if F1 and F2 containing PPE were able to maintain the AA potential of PPE raw material, DPPH, ABTS, and FRAP assays were performed with F1 and F2 containing PPE and the results were compared to ethanolic solution of PPE in the same concentration in the reaction medium. Figure 3a shows that the capacity to scavenge DPPH radical maintained in the formulations with different content of lipidic (43.55%, 48.23%, and 45.63% for PPE, F1, and F2, respectively). However, there was a significant decrease in AA of F1 and F2 containing PPE compared to PPE raw material when measured by FRAP and ABTS methods. The percentages of reduction of ABTS radical were 53.10%, 44.99%, and 46.52% for PPE, F1, and F2, respectively (Fig. 3b). In FRAP assay, the reducing power was 0.051, 0.039, and 0.041 μmol/L trolox equivalent/μg/mL of extract for PPE, F1, and F2, respectively (Fig. 3c). These results demonstrated that there was a reduction of 15.27% and 22.93% of the ABTS scavenging activity of F1 and F2, respectively, and of 12.39% and 18.83% of the iron-reducing power of F1 and F2, respectively.
Fig. 3.

Evaluation of the AA of PPE and both formulations containing PPE: H-donor ability of PPE using stable radical DPPH (a), scavenging ability of PPE using ABTS method (b), and ferric-reducing antioxidant power using FRAP test (c)*Significant statistical difference compared to PPE raw material (P<0.05)
Stability Studies
F1 and F2 maintained their color and consistency characteristics under RT and 4°C. However, a gradual color change of F1 and mainly of F2 under 40 ± 2°C/75 ± 5% RH was observed. In general, the pH values of F1 and F2 remained compatible with the skin and both formulations also remained physically stable, showing no phase separation (Table II). Regarding functional stability, the hydrogen-donating ability was kept in both formulations at RT and 4°C. However, after 6 months stored at 40 ± 2°C/75 ± 5% RH, F1, and F2 lost approximately 7.23% and 21.76% of its AA, respectively (Fig. 4).
Table II.
Physico-chemical Characteristic of F1 and F2 Containing or Not P. pseudocaryophyllus Ethanolic Extract
| Formulation | pH | Centrifugation |
|---|---|---|
| F1 control | 4.67 | NS |
| F1 + PPE | 4.65 | NS |
| F2 control | 6.65 | NS |
| F2 + PPE | 6.46 | NS |
NS no separation
Fig. 4.

Stability of hydrogen-donation ability of PPE (a), F1 (b), and F2 (c) containing PPE stored at 4°C, RT, and 40°C/75% RH for 6 months
In Vitro Release Studies
Figure 5 shows the PPE release (%) over time ± SEM of F1 and F2. The maximal antioxidant activity observed for formulations 1 and 2 after 12 h of experiment was 14.66% and 13.41%, respectively, without significant statistical difference between the formulations. Furthermore, a gradual increase in the radical scavenge activity over time (3, 6, 9, and 12 h) was detected with F1 and F2 containing PPE. Statistical significant differences were detected after 9 and 12 h compared to 3 h, and after 12 h compared to 3, 6, and 9 h. At 12 h there was no statistical difference between F1 and F2.
Fig. 5.
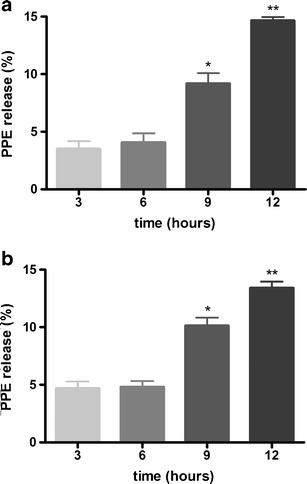
Percentage of PPE release measured by hydrogen-donating ability as a function of the time from F1 (a) and F2 (b). Results are represented by means ± SEM (n = 5). Statistical analysis was performed by one-way ANOVA followed by Bonferroni’s test of multiple comparisons. *Significant statistical difference compared to 3 h (P < 0.05). **Significant statistical difference compared to 3, 6, and 9 h (P < 0.05)
In Vivo Studies
In this study, UV-B irradiation induced a decrease of approximately 1.58- and 1.84-fold of ABTS scavenging capacity and iron-reducing power of the skin, respectively. Both formulations containing PPE inhibited this depletion, maintaining levels similar to control (non-irradiated) group in both tests (Fig. 6).
Fig. 6.

Formulations containing PPE increases the antioxidant capacity of skin after UV-B irradiation in ABTS assay (a) and in ferric reducing antioxidant power (b). Bars represent means ± SEM of two separated experiments, five mice per group. *P < 0.001 compared to the control (non-irradiated) group, **P < 0.001 compared to irradiated group and F1 control group, and ***P < 0.001 compared to irradiated group and F2 control group
DISCUSSION
Antioxidants obtained from natural sources may provide new possibilities for the treatment and prevention of oxidative stress-mediated diseases; therefore plant extracts rich in antioxidant compounds have gained special attention (29). As described above, P. pseudocaryophyllus has been used as medicinal plant in folk medication system and is rich in bioactive compounds responsible for that properties (11,12). In this study, we have found that polyphenols content was approximately seven times higher than total of flavonoids content of PPE, corroborating other studies that show that P. pseudocaryophyllus contains not only flavonoids, but other polyphenolic substances, like tannins (12,13). Previously, Paula et al. (13), and Fajemiroye et al. (12) have reported that this plant contains flavonoids and phenolic compounds. Some compounds were isolated as flavonoids and phenolic acids derivatives, and in the literature eugenol is reported as the main compound of P. pseudocaryophyllus (30). In this study, eugenol, rutin, and tannic acid were identified in the extract, and these substances may have exerted synergistic effect. Despite eugenol being in greater quantity, it may be suggested that tannic acid and rutin are mainly responsible for the antioxidant activity o PPE.
Two or more methods should always be employed to evaluate the antioxidant potential of any substance, once oxidative stress depends on the type of ROS generated, how and where it is generated, and the oxidative target evaluated. Furthermore, plant extracts present a diversified phytochemical composition, therefore its AA may be attributed to synergistic action of multiple substances, which belong to different chemical groups and may exert its activity through various mechanisms (13,31). Thus, the AA of PPE was evaluated by three different methods. The ABTS and DPPH methods consist on the verification of the colored radical suppression on the medium, which decreases the absorbance. In FRAP assay, antioxidants in the sample reduce the Fe+3–TPTZ complex to form a blue-colored Fe+2–TPTZ complex, which results in an increase in the absorbance. P. pseudocaryophyllus components act as free radical scavengers of the negatively and positively charged free radicals, DPPH and ABTS, respectively. Furthermore, P. pseudocaryophyllus also demonstrated ferric-reducing antioxidant power.
The water-soluble vitamin C is a well-known preventive and chain-breaking antioxidant. Experiments show that the antioxidant activity of vitamin C involves a hydrogen transfer rather than an electron transfer. In vitro, vitamin C behaves as an efficient antioxidant in several different ways such as reducing lipid peroxidation and scavenging peroxyl, thiyl, sulphenyl, urate, nitroxide, and other radicals (32). Thus, we conducted the evaluation of scavenging activity of ABTS and DPPH and iron reduction capacity of vitamin C as a standard to compare with the results for PPE. The IC50 values for vitamin C were 3.0 and 2.5 μg/mL in the DPPH and ABTS assays, respectively. These results were close to those observed with PPE considering the total solids in the extract, which demonstrates the relevance of free radical scavenging activity of PPE. Nevertheless, the ferric-reducing antioxidant ability of PPE was lower than that of vitamin C (12.23 μMol/L trolox/μg/mL).
Active molecules in extracts present varied chemical and physical characteristics to be considered in the development of pharmaceutical formulations. Furthermore, fluid and semi-solid emulsions are used as vehicles to pharmaceutical products for skin release and their coloidal properties influence drug bioavailability (29). In this sense, it is important to use formulations with different proportions of lipidic and aqueous constituents to determine AA and in vitro release of active molecules. The excipients used in the preparation of formulations F1 and F2 were chosen based on the previous studies of our group (13,20,26,29,33).
In order to verify if F1 and F2 constituents were able to maintain the antioxidant potential of PPE, the same tests used to evaluate the AA of the extract were performed with the formulations containing PPE. The capacity to scavenge DPPH radical was maintained, but FRAP and ABTS assays showed a decrease in the AA of PPE after its incorporation in topical formulations. This may be explained by the fact that the antioxidant compounds present in the extract may interact with components of the vehicle and the fatty phase of emulsions (34). Therefore, it can be suggested that the colloidal properties of self-emulsifying base Polawax® used in both formulations could promote interaction with the components of PPE which are responsible for the reducing power and/or with the components capable of donating electrons to ABTS. In addition, the interference of formulation components might occur in the development of the reactions involved with antioxidant methods as well as the possible inhibition of this activity by these components. This raises concerns about formulations, since one of most challenging tasks in evaluating topical formulations is to deal with the presence of the compounds present in formulations that may cause interference if using a non-specific method (14). Therefore, the use of varied methodologies to evaluate in vitro the maintenance of AA of active principles added in topical formulations is important, mainly, in the case of vegetal extracts in which the active principles act in synergy and can interact differentially with the vehicle that is also of complex composition.
There was no interference of the formulations in stable free radical DPPH assay, which means that this method can be used to evaluate the AA of formulations. For this reason, this assay was used for evaluating the functional stability of PPE extract and it added in formulations.
A stable emulsion maintains the proper proportions between its components and the interphase surface even after being exposed to tension resulting from factors such as temperature, agitation, and acceleration of gravity (35). Thus, two emulsions containing PPE were developed and their physico-chemical and functional stability were evaluated at predetermined times. The formulations developed in this study presented different characteristics, mainly in their lipid content, so several physical instabilities could occur when the complex compounds present in the PPE were added. Therefore, stability testing represents a crucial part of the testing program since the instability of the product modifies essential requisites, i.e., quality, efficacy, and safety (36).
During the study, both emulsions remained physically stable and pH values remained satisfactory, which ensures that F1 and F2 are compatible with the application site, avoiding irritation (24). Regarding functional stability study of DPPH scavenging activity, it was observed that temperature, storage time, and type of formulation influenced the AA of PPE. Hydrolysis reactions are one of the most common processes of active components degradation, and depend mainly on the temperature and quantity of available water in the medium (37). Since F2 showed a loss of AA approximately three times higher than F1, it can be suggested that the higher water content of F2 coupled with drastic conditions of storage destabilized active compounds of PPE. The decrease in the AA of formulations stored at accelerated conditions corroborates the results of visual evaluation, which showed changes in color of formulations, especially of F2. Polyphenols are susceptible to the action of temperature and humidity, and its stability profile and biological activity are strongly related to the processing conditions and storage (38). Thus, the reduction in AA observed in formulations stored under accelerated conditions may be related to a possible degradation of polyphenols present in the extract. We have also determined the particle size distributions of the formulations using LS 13 320 Laser Diffraction Particle Size Analyzer (Beckman Coulter). The results showed the values: F1 without PPE, 40.13 μm; F1 added PPE, 30.59 μm; F2 without PPE, 15.66 μm; and F2 added PPE, 9.23 μm. We also determined the particle size dispersal coefficient, Span (39) and the results showed that all the studied formulations were polydisperse systems (data not shown).
It is generally assumed that the nature of the delivered pharmaceutical dosage strongly influences the rate and extent of drug release. Release may be improved by selecting the appropriate vehicle. The in vitro release studies which measure drug/vehicle interactions are considered to be useful and crucial in pre-formulation step to choose an appropriate vehicle (40,41). The release of antioxidant compounds of the PPE from different emulsion systems (F1 and F2) through nitrocellulose membrane was examined and the values found were very close to both formulations, showing that the difference in lipid content did not affect the release of these components of the extract.
Regarding in vivo studies, we evaluated the effectiveness of F1 and F2 incorporated with PPE against oxidative damage caused by UV-B irradiation. Once oxidative stress is characterized by the decrease of endogenous antioxidant, several methods have been developed to assess the antioxidant capacity of diverse organs (2,42). The difficulty in measuring each antioxidant component separately and the interactions among them leads to the use of quick, simple, and efficient assays, like ABTS and FRAP, which use different principles to measure antioxidant capacity (28).
Corroborating the release studies that demonstrated similar results for both formulations, the treatment with these topical formulations containing PPE clearly improved the cutaneous antioxidant capacity to control levels. ABTS assay has been found to correlate well with levels of endogenous glutathione (43), while FRAP assay may reflect levels of ascorbic acid, uric acid, and α-tocopherol (28).
It is noteworthy to mention that hairless mice were also observed during 72 h after topical application of F1 or F2 containing or not PPE, and no erythema was visually evident. In the acute toxicity test with fixed doses (5, 50, 300, and 2,000 mg/kg) accordingly with the OECD guidelines no 420 for testing of chemicals, there was no lethality or weight loss up to the dose of 2,000 mg/kg and 14 days of evaluation (group’s unpublished observation). In agreement, P. pseudocaryophyllus leaves are used to prepare tea in Brazil (44). Therefore, P. pseudocaryophyllus seems safe for therapeutic purposes.
Despite the need for further studies, the prepared formulations containing PPE demonstrate interesting attributes to be explored as potential products to be used against UV-induced damages.
CONCLUSIONS
For the DPPH and ABTS assays the PPE showed a dose-dependent activity, and showed small values of IC50 demonstrating ability to scavenging cation and anion radicals. In addition, PPE also showed iron reduction capacity by FRAP method. Therefore, these methods are adequate to evaluate the AA of PPE. However, only DPPH assay was adequate to evaluate the maintenance of the AA of PPE added F1 and F2. Nevertheless, it is essential to choose the correct method to evaluate the antioxidant activity of these formulations to perform stability studies. During 6 months of the study in different storage conditions, F1 and F2 added or not with PPE were stable to physico-chemical tests. The evaluation of the radical scavenging activity of PPE incorporated in formulations did not demonstrate loss of activity by storage at 4°C/6 months. Nevertheless, both formulations lost AA activity at 40°C ± 2°C/75 ± 5 RH, and F2 present a 3-fold higher loss of AA activity compared to F1. In addition, both formulations were able to gradually release the antioxidant compounds present in the PPE reaching maximal release at 12 h without statistical differences between F1 and F2. Pretreatment with PPE added F1 or F2 significantly improved the cutaneous antioxidant capacity to control levels after UV-B irradiation. Therefore, the present results suggest that formulations containing PPE might be a conceivable topical source of antioxidant compounds that decrease oxidative damages of the skin.
ACKNOWLEDGMENTS
This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and SETI/Fundação Araucária and Paraná State Government. We thank Denise Duarte from Post-graduation Laboratory of UEL and José Orestes Del Ciampo from Faculdade de Ciências Farmacêuticas-USP for the technical assistance.
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Marcela Z. Campanini, Phone: +55-43-33712475
Ana L. M. Ivan, Phone: +55-43-33712475
Sarah M. Martins, Phone: +55-43-33712475
Maria J. R. Paranzini, Phone: +55-43-33712475
Renata M. Martinez, Phone: +55-43-33712475
Nilton S. Arakawa, Phone: +55-43-33712475
Marcela M. Baracat, Phone: +55-43-33712475
Rúbia Casagrande, Phone: +55-43-33712475, Email: rubiacasa@yahoo.com.br.
Sandra R. Georgetti, Phone: +55-43-33712475, Email: sangeorgetti@gmail.com
REFERENCES
- 1.Al Shaal L, Shegokar R, Müller RH. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int J Pharm. 2011;420:133–140. doi: 10.1016/j.ijpharm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. The wanderings of a free radical. Free Radic Biol Med. 2009;46:531–542. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Vicentini FTMC, He T, Shao Y, Fonseca MJV, Verri WA, Jr, et al. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-κB pathway. J Dermatol Sci. 2011;61:162–168. doi: 10.1016/j.jdermsci.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Autier P, Dore JF, Cattaruzza MS, Renard F, Luther H, Gentiloni-Silverj F. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst. 1998;90:1873–1880. doi: 10.1093/jnci/90.24.1873. [DOI] [PubMed] [Google Scholar]
- 5.Azizi E, Iscovich J, Pavlotsky F, Shafir R, Luria I, Federenko L. Use of sunscreen is linked with elevated naevi counts in Israeli school children and adolescents. Melanoma Res. 2000;10:491–498. doi: 10.1097/00008390-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Bauer J, Buttner P, Wiecker TS, Luther H, Garbe C. Effect of sunscreen and clothing on the number of melanocytic nevi in 1,812 German children attending day care. Am J Epidemiol. 2005;161:620–627. doi: 10.1093/aje/kwi086. [DOI] [PubMed] [Google Scholar]
- 7.Verschooten L, Claerhout S, Van Laethem A, Agostinis P, Garmyn M. New strategies of photoprotection. Photochem Photobiol. 2006;82:1016–1023. doi: 10.1562/2006-04-27-IR-884.1. [DOI] [PubMed] [Google Scholar]
- 8.Atoui AK, Mansouri A, Boskou G, Kefalas P. Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chem. 2005;89:27–36. doi: 10.1016/j.foodchem.2004.01.075. [DOI] [Google Scholar]
- 9.Fonseca YM, Catini CD, Vicentini FTMC, Nomizo A, Gerlach RF, Fonseca MJV. Protective effect of Calendula officinalis extract against UV-B-induced oxidative stress in skin: evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J Ethnopharmacol. 2010;127:596–601. doi: 10.1016/j.jep.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Verri WA, Jr, Vicentini FTMC, Baracat MM, Georgetti SR, Cardoso RDR, Cunha TM, et al. Flavonoids as anti-inflammatory and analgesic drugs: mechanisms of action and perspectives in the development of pharmaceutical forms. In: Atta-ur-Rahman FRS, et al., editors. Studies in natural products chemistry. Amsterdam: Elsevier; 2012. pp. 297–330. [Google Scholar]
- 11.Paula JAM, Paula JR, Bara MTF, Rezende MH, Ferreira HD. Estudo farmacognóstico das folhas de Pimenta pseudocaryophyllus (Gomes) L.R. Landrum—Myrtaceae. Rev Bras Farmacogn. 2008;18:265–278. doi: 10.1590/S0102-695X2008000200022. [DOI] [Google Scholar]
- 12.Fajemiroye JO, Galdino PM, Alves SF, de Paula JAM, de Paula JR, Ghedini PC, et al. Involvement of 5-HT1A in the anxiolytic-like effect of dichloromethane fraction of Pimenta pseudocaryophyllus. J Ethnopharmacol. 2012;141:872–877. doi: 10.1016/j.jep.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Georgetti SR, Casagrande R, Vicentini FTMC, Verri WA, Jr, Fonseca MJV. Evaluation of the antioxidant activity of soybean extract by different in vitro methods and investigation of this activity after its incorporation in topical formulations. Eur J Pharm Biopharm. 2006;64:99–106. doi: 10.1016/j.ejpb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Marquele FD, Di Mambro VM, Georgetti SR, Casagrande R, Valim YML, Fonseca MJ. Assessment of the antioxidant activities of Brazilian extracts of propolis alone and in topical pharmaceutical formulations. J Pharm Biomed. 2005;39:455–462. doi: 10.1016/j.jpba.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Marquele FD, de Oliveira ARM, Bonato PS, Lara MG, Fonseca MJV. Própolis extract release evaluation from topical formulations by chemiluminescence and HPLC. J Pharm Biomed. 2006;41:461–468. doi: 10.1016/j.jpba.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Vicentini FTMC, Georgetti SR, Bentley MVLB, Fonseca MJV. Assessment of in vitro methodologies to determine topical and transdermal delivery of the flavonoid Quercetin. Braz J Pharm Sci. 2009;45:357–364. doi: 10.1590/S1984-82502009000200022. [DOI] [Google Scholar]
- 17.Kumatzawa S, Hamasaka T, Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004;84:329–339. doi: 10.1016/S0308-8146(03)00216-4. [DOI] [Google Scholar]
- 18.Georgetti SR, Casagrande R, Verri WA, Jr, Borin MF, Rafael JA, Jabor JR, et al. Assessment of the antioxidant activity of two plant extracts containing isoflavonoids by different in vitro methods. Lat Am J Pharm. 2007;26:252–257. [Google Scholar]
- 19.Rieger SC. Constituintes químicos e atividades antioxidante, bacteriostática e anti-helmíntica de Inga marginata Willd [dissertation] Londrina: Universidade Estadual de Londrina; 2011. [Google Scholar]
- 20.Casagrande R, Georgetti SR, Verri WA, Jr, Borin MF, Lopez RFV, Fonseca MJV. In vitro evaluation of quercetin cutaneous absorption from topical formulations and its functional stability by antioxidant activity. Int J Pharm. 2007;328:183–190. doi: 10.1016/j.ijpharm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 22.Munteanu FD, Basto C, Gubitz GM, Cavaco-Paulo A. Staining of wool using the reaction products of ABTS oxidation by Laccase: synergetic effects of ultrasound and cyclic voltammetry. Ultrason Sonochem. 2007;14:363–367. doi: 10.1016/j.ultsonch.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez-Gonzalez I, Jiménez-Escrig A, Saura-Calixto F. In vitro antioxidant activity of coffees brewed using different procedures (Italian, espresso and filter) Food Chem. 2005;90:133–139. doi: 10.1016/j.foodchem.2004.03.037. [DOI] [Google Scholar]
- 24.Casagrande R, Baracat MM, Georgetti SR, Verri WA, Jr, Vicentini FTMC, Rafael JA, et al. Method validation and stability study of quercetin in topical emulsions. Quim Nova. 2009;32:1939–1942. doi: 10.1590/S0100-40422009000700041. [DOI] [Google Scholar]
- 25.Anchisi C, Maccioni AM, Sinico C, Valenti D. Stability studies of new cosmetic formulations with vegetable extracts as functional agents. IL Farmaco. 2001;56:427–431. doi: 10.1016/S0014-827X(01)01055-2. [DOI] [PubMed] [Google Scholar]
- 26.Casagrande R, Georgetti SR, Verri WA, Jr, Dorta DJ, Santos AC, Fonseca MJV. Protective effect of topical formulations containing quercetin against UV-B-induced oxidative stress in hairless mice. J Photochem Photobiol B. 2006;84:21–27. doi: 10.1016/j.jphotobiol.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Shindo Y, Witt E, Han D, Packer L. Dose–response effects of acute ultraviolet irradiation on antioxidants and molecular markers of oxidation in murine epidermis and dermis. J Investig Dermatol. 1994;102:470–475. doi: 10.1111/1523-1747.ep12373027. [DOI] [PubMed] [Google Scholar]
- 28.Katalinic V, Modun D, Music I, Boban M. Gender differences in antioxidant capacity of rat tissues determined by 2,2V-azinobis (3-ethylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comp Biochem Physiol C. 2005;140:47–52. doi: 10.1016/j.cca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Marquele-Oliveira F, Fonseca YM, de Freitas O, Fonseca MJV. Development of topical functionalized formulations added with própolis extract: stability, cutaneous absorption and in vivo studies. Int J Pharm. 2007;342:40–48. doi: 10.1016/j.ijpharm.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 30.Paula JAM, Silva MRR, Costa MP, Diniz DGA, Sá FAS, Alves SF, et al. Phytochemical analysis and antimicrobial, antinociceptive, and anti-inflammatory activities of two chemotypes of Pimenta pseudocaryophyllus (myrtaceae) Evid Based Complement Alternat Med. 2012 doi: 10.1155/2012/420715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sousa CMM, Silva HR, Vieira GMJ, Ayres MCC, Costa CLS, Araújo DS, et al. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quim Nova. 2007;30:351–355. doi: 10.1590/S0100-40422007000200021. [DOI] [Google Scholar]
- 32.Drach M, Narkiewicz-Michalek J, Sienkiewicz A, Szymula M, Bravo-Díaz C. Antioxidative properties of vitamins C and E in micellar systems and in microemulsions. Colloid Surface A. 2011;379:79–85. doi: 10.1016/j.colsurfa.2010.11.073. [DOI] [Google Scholar]
- 33.Georgetti SA, Casagrande R, Verri WA, Jr, Lopes RFV, Fonseca MJV. Evaluation of in vivo efficacy of topical formulations containing soybean extract. Int J Pharm. 2008;352:189–196. doi: 10.1016/j.ijpharm.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 34.Di Mambro VM, Fonseca MJV. Assessment of physical and antioxidant activity stability, in vitro release and in vivo efficacy of formulations added with superoxide dismutase alone or in association with a-tocopherol. Eur J Pharm Biopharm. 2007;66:451–459. doi: 10.1016/j.ejpb.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Pather SI, Neau SH, Pather S. A comparison of two quality assessment methods for emulsions. J Pharm Biomed Anal. 1995;13:1283–1289. doi: 10.1016/0731-7085(95)01533-Q. [DOI] [PubMed] [Google Scholar]
- 36.Bilia AR, Bergonzi MC, Morgenni F, Mazzi G, Vincieri FF. Evaluation of chemical stability of St. John’s wort commercial extract and some preparations. Int J Pharm. 2001;213:199–208. doi: 10.1016/S0378-5173(00)00660-8. [DOI] [PubMed] [Google Scholar]
- 37.Waterman KC, Adami RC. Accelerated aging: prediction of chemical stability of pharmaceuticals. Int J Pharm. 2005;293:101–125. doi: 10.1016/j.ijpharm.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Ungar Y, Oluwatooyin F, Shimoni E. Thermal stability of genistein and daidzein and its effect on their antioxidant activity. J Agric Food Chem. 2003;51:4394–4399. doi: 10.1021/jf034021z. [DOI] [PubMed] [Google Scholar]
- 39.Xiao X-C, Chu L-Y, Chen W-M, Zhu J-H. Monodispersed thermoresponsive hydrogel microspheres with a volume phase transition driven by hydrogen bonding. Polymer. 2005;46:3199–3209. doi: 10.1016/j.polymer.2005.01.075. [DOI] [Google Scholar]
- 40.Ozsoy Y, Güngör S, Cevher E. Vehicle effects on in vitro release of tiaprofenic acid from different topical formulations. IL Farmaco. 2004;59:563–566. doi: 10.1016/j.farmac.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Röpke CD, Kaneko TM, Rodrigues RM. Evaluation of percutaneous absorption of 4-nerolidylcathecol from four topical formulations. Int J Pharm. 2002;249:109–116. doi: 10.1016/S0378-5173(02)00477-5. [DOI] [PubMed] [Google Scholar]
- 42.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 43.Kang HM, Saltveit M. Antioxidant capacity of lettuce leaf tissue increases after wounding. J Agric Food Chem. 2002;50:7536–7541. doi: 10.1021/jf020721c. [DOI] [PubMed] [Google Scholar]
- 44.Lorenzi H, Matos FJ. Plantas Medicinais no Brasil, nativas e exóticas. Nova Odessa: Instituto Plantarum; 2008. p. 576. [Google Scholar]


