Figure 7.
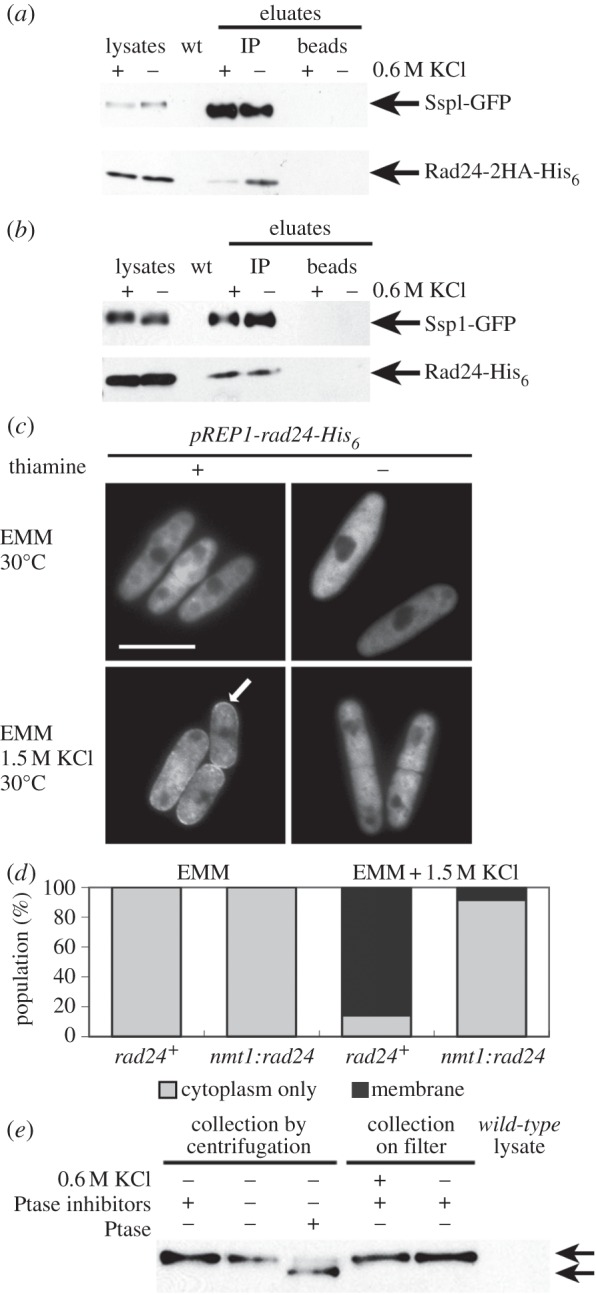
Treatment with 0.6 M KCl for 15 min reduces Rad24-2HA-His6 co-immunoprecipitation with Ssp1-GFP. Cells were co-expressing Ssp1-GFP and Rad24-2HA-His6 (a) or Ssp1-GFP and Rad25-His6 (30°C, YEA). YEA + KCl to 0.6 M (30°C) (b) was added to aliquots of cells. Ssp1-GFP (5–10 μg), Rad24-2HA-His6 (2.5–10 μg) and Rad25-His6 (15 μg) were detected in the cell lysates used for the immunoprecipitation. (c,d) Overexpression of rad24-His6 reduces Ssp1-GFP cell membrane localization after 0.6 M KCl treatment. (c) A plasmid producing Rad24-His6 under the control of the nmt1 promoter was expressed in ssp1-GFP:kanRint (30°C) in EMM (–thiamine) for 19 h. Images were taken 10–15 min after 1.5 M KCl stress. (d) Single-copy nmt1:GFP-ssp1 was overexpressed in either a rad24+ background or co-overexpressed with the single-copy integrant nmt1:rad24−His6 in the absence of thiamine for 20 h (30°C). (e) Phosphorylation state of Ssp1-GFP in YEA and YEA + 0.6 M KCl. Cell extracts were prepared in the presence (lane 1) and absence (lane 2) of phosphatase inhibitors. Phosphatase-inhibitor-free Ssp1-GFP extracts (5 μg) were treated with Lambda phosphatase as indicated. Cells were collected on a filter after treatment with YEA or YEA to 0.6 M KCl (30°C). Upper arrow denotes Ssp1-GFP before treatment with Lambda phosphatase.
