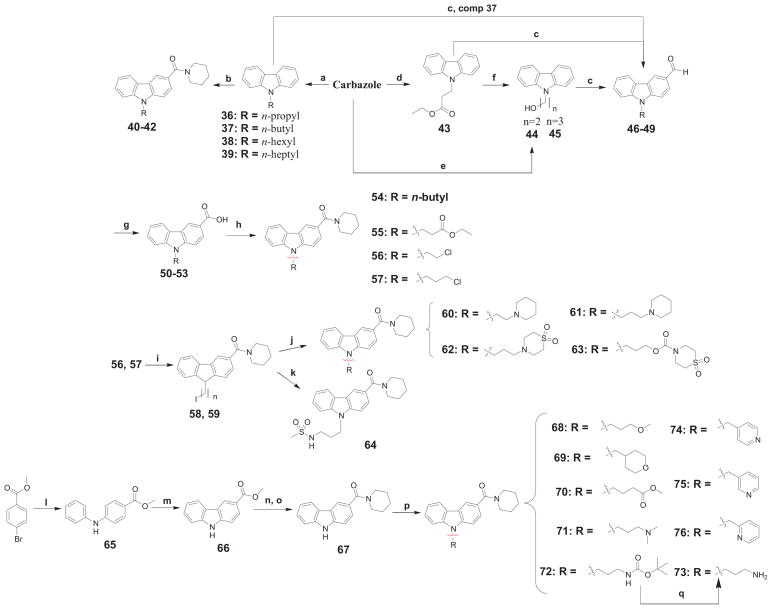
Scheme 4.
Synthesis of (9-substituted-9H-carbazol-3-yl)(piperidin-1-yl)methanone derivatives 54-57, 60-64 and 68-76.
a Reagents and conditions: (a) n-alkyl bromide or iodide, NaOH, acetone, reflux, 16h; (b) AlCl3, piperidinecarbonyl chloride, benzene, 0°C → μw at 100°C, 1h; (c) DMF, POCl3, 0°C → μw at 100°C, 1h; (d) ethyl acrylate, K2CO3, DMF, rt, 19h; (e) ethylene oxide, KOH, methyl vinyl ketone, μw at 50°C, 1h; (f) LAH, THF, 0°C → rt, 1h; (g) KMnO4, acetone-water/acetic acid, 0°C → reflux, 3h; (h) DIEA, DMAP, piperidine, EDAC·HCl, DCM, 0°C → rt, 16h; (i) NaI, acetonitrile, reflux, 72h; (j) corresponding amine, Cs2CO3, acetone, rt → 80°C, 1 h; (k) NaH, DMF, 0°C → rt, 16h; (l) aniline, 5 mol% Pd(OAc)2, 5 mol% rac-BINAP, K2CO3, toluene, μW at 160°C, 1h; (m) Pd(OAc)2, AcOH, reflux, 1 h; (n) KOH, EtOH/H2O, reflux, 16 h; (o) DIEA, DMAP, piperidine, EDAC·HCl, DCM, 16h; (p) corresponding substituted alkyl bromide, TBAI, Cs2CO3, DMF, μW at 140°C, 2 h, or KOt-Bu, rt, 16 h; (q) gaseous HCl, EtOAc, 0°C → rt.