Abstract
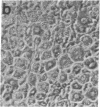
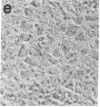
Differentiating mouse 3T3-L1 preadipocytes have been used as a model system to study the ability of type beta transforming growth factor (TGF-beta) to modulate cell development. We find that TGF-beta inhibits potently (ID50 approximately equal to 25 pM) the adipogenic conversion of 3T3-L1 cells. Inhibition is observed only when cells are exposed to TGF-beta before they become committed to differentiation. Even a transient (4 hr) exposure to TGF-beta immediately before the commitment point is sufficient to prevent differentiation. This point coincides with the time point immediately preceding the onset of coordinate expression of differentiation-specific proteins in 3T3-L1 cells. TGF-beta interacts with cell surface receptors in 3T3-L1 cells that have structural and binding properties similar to TGF-beta receptors in other cell types in which TGF-beta acts as a growth activator or a growth inhibitor. However, TGF-beta does not markedly alter differentiation-related mitosis in 3T3-L1 cells. The action of TGF-beta on 3T3-L1 cells does not involve changes in cAMP or prostaglandin E levels. These results suggest that TGF-beta is a unique modulator of adipogenic differentiation of fibroblasts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Grotendorst G. R., Miller D. M., Sporn M. B. Cellular transformation by coordinated action of three peptide growth factors from human platelets. 1984 Jun 28-Jul 4Nature. 309(5971):804–806. doi: 10.1038/309804a0. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Bernlohr D. A., Angus C. W., Lane M. D., Bolanowski M. A., Kelly T. J., Jr Expression of specific mRNAs during adipose differentiation: identification of an mRNA encoding a homologue of myelin P2 protein. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5468–5472. doi: 10.1073/pnas.81.17.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr D. A., Bolanowski M. A., Kelly T. J., Jr, Lane M. D. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1985 May 10;260(9):5563–5567. [PubMed] [Google Scholar]
- Brown D. D. The role of stable complexes that repress and activate eucaryotic genes. Cell. 1984 Jun;37(2):359–365. doi: 10.1016/0092-8674(84)90366-0. [DOI] [PubMed] [Google Scholar]
- Burstein S., Hunter S. A., Sedor C., Shulman S. Prostaglandins and cannabis--IX. Stimulation of prostaglandin E2 synthesis in human lung fibroblasts by delta 1-tetrahydrocannabinol. Biochem Pharmacol. 1982 Jul 15;31(14):2361–2365. doi: 10.1016/0006-2952(82)90530-5. [DOI] [PubMed] [Google Scholar]
- Childs C. B., Proper J. A., Tucker R. F., Moses H. L. Serum contains a platelet-derived transforming growth factor. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5312–5316. doi: 10.1073/pnas.79.17.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolik C. A., Wakefield L. M., Smith D. M., Sporn M. B. Characterization of a membrane receptor for transforming growth factor-beta in normal rat kidney fibroblasts. J Biol Chem. 1984 Sep 10;259(17):10995–11000. [PubMed] [Google Scholar]
- Hayashi I., Nixon T., Morikawa M., Green H. Adipogenic and anti-adipogenic factors in the pituitary and other organs. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3969–3972. doi: 10.1073/pnas.78.6.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. L., Deykin D. The activation of phosphatidylinositol-hydrolyzing phospholipase A2 during prostaglandin synthesis in transformed mouse BALB/3T3 cells. J Biol Chem. 1981 May 25;256(10):5215–5219. [PubMed] [Google Scholar]
- Kuri-Harcuch W., Green H. Increasing activity of enzymes on pathway of triacylglycerol synthesis during adipose conversion of 3T3 cells. J Biol Chem. 1977 Mar 25;252(6):2158–2160. [PubMed] [Google Scholar]
- Kuri-Harcuch W., Green H. Suppression of the adipose conversion of 3T3 cells by acidified serum. J Cell Physiol. 1981 Sep;108(3):455–460. doi: 10.1002/jcp.1041080320. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Student A. K., Polakis S. E., Lane M. D. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976 Oct 25;251(20):6462–6464. [PubMed] [Google Scholar]
- Massagué J., Kelly B., Mottola C. Stimulation by insulin-like growth factors is required for cellular transformation by type beta transforming growth factor. J Biol Chem. 1985 Apr 25;260(8):4551–4554. [PubMed] [Google Scholar]
- Massagué J., Like B. Cellular receptors for type beta transforming growth factor. Ligand binding and affinity labeling in human and rodent cell lines. J Biol Chem. 1985 Mar 10;260(5):2636–2645. [PubMed] [Google Scholar]
- Pairault J., Green H. A study of the adipose conversion of suspended 3T3 cells by using glycerophosphate dehydrogenase as differentiation marker. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5138–5142. doi: 10.1073/pnas.76.10.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Cyclic AMP-mediated control of lipogenic enzyme synthesis during adipose differentiation of 3T3 cells. Cell. 1981 May;24(2):503–510. doi: 10.1016/0092-8674(81)90341-x. [DOI] [PubMed] [Google Scholar]
- Tucker R. F., Branum E. L., Shipley G. D., Ryan R. J., Moses H. L. Specific binding to cultured cells of 125I-labeled type beta transforming growth factor from human platelets. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6757–6761. doi: 10.1073/pnas.81.21.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- Williams I. H., Polakis S. E. Differentiation of 3T3-L1 fibroblasts to adipocytes. The effect of indomethacin, prostaglandin E1 and cyclic AMP on the process of differentiation. Biochem Biophys Res Commun. 1977 Jul 11;77(1):175–186. doi: 10.1016/s0006-291x(77)80180-0. [DOI] [PubMed] [Google Scholar]
- Wise L. S., Sul H. S., Rubin C. S. Coordinate regulation of the biosynthesis of ATP-citrate lyase and malic enzyme during adipocyte differentiation. Studies on 3T3-L1 cells. J Biol Chem. 1984 Apr 25;259(8):4827–4832. [PubMed] [Google Scholar]