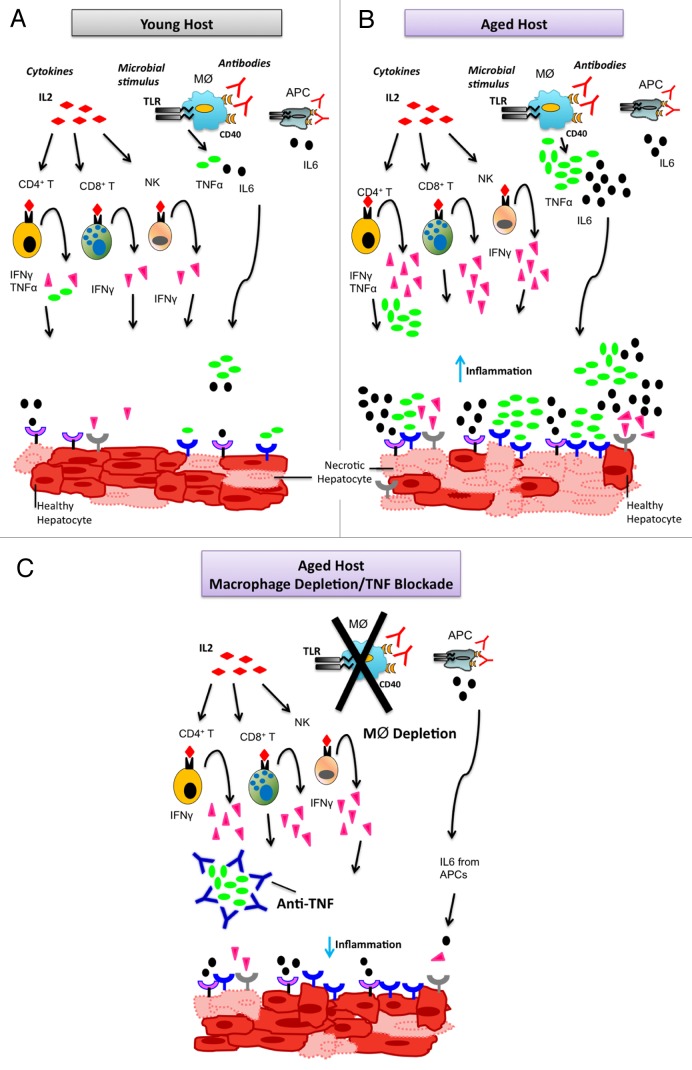
Figure 1. Strategy to circumvent potentially lethal side effects of cancer immunotherapy in the elderly. (A–C) Immunomodulatory agents including cytokines, such as interleukin (IL)-2, microbial components that operate as Toll-like receptor (TLR) agonists, and immunostimulatory antibodies, such as CD40-targeting antibodies, lead to the systemic secretion of pro-inflammatory cytokines. IL-2 leads to the production of interferon γ (IFNγ) by CD4+ T cells, CD8+ T cells and natural killer (NK) cells, as well as to the secretion of tumor necrosis factor α (TNFα) by CD4+ T cells in both young (A) and aged (B) hosts. Microbial components and immunostimulatory antibodies activate macrophages (MØ) and other antigen-presenting cells (APCs) to produce TNFα and IL-6 in both young (A) and aged (B) animals. However, the administration of immunostimulatory agents to aged hosts results in the massive secretion of pro-inflammatory cytokines, leading to multi-organ failure (B). Immunotherapeutic interventions result in a robust inflammatory responses and extensive hepatic necrosis in aged (B), but not young (A) mice. The neutralization of TNFα by specific antibodies or macrophage depletion limits the secretion of pro-inflammatory cytokines by aged hosts responding to immunotherapy, hence inhibiting systemic and local inflammation, reducing hepatic necrosis and ultimately protecting these animals from immunotherapy-associated lethality.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.