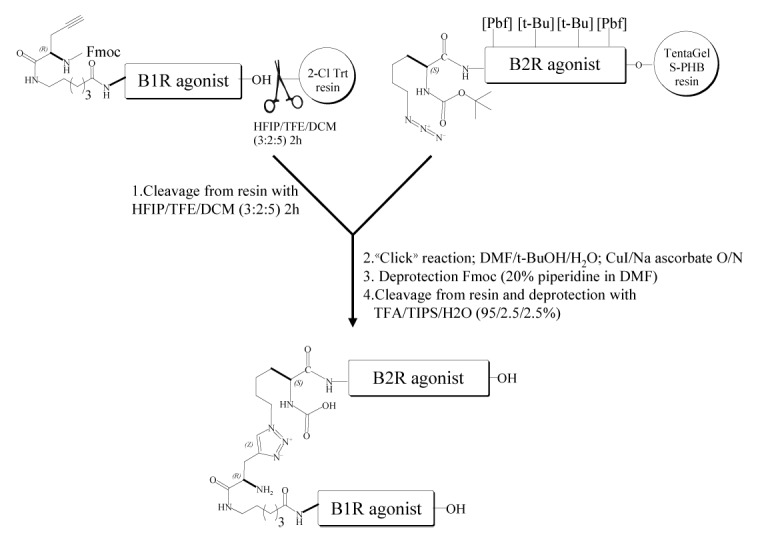
Scheme 1. Construction of a Nα-linked B1R/B2R agonist heterodimer utilizing “click” chemistry and Aca linker. Chemistry conjugation reaction between an alkyne-containing B1R agonist molecule (Fmoc-G(propargyl)-Aca-Lys-Arg-Pro-Pro-Gly-Phe-Ser-Pro-DPhe-OH) and an azide-containing B2R agonist molecule (Boc-Lys(N3)-Arg(Pdf)-Pro-Hyp(t-Bu)-Gly-Thi-Ser(t-Bu)-NChg-Thi-Arg(Pbf)). The peptide B1R agonist (in solution) and the peptide B2R agonist (immobilized on the solid-phase) were then joined at their N-terminal side via a triazole linker, made by “click” chemistry. Heterodimer was then deprotected, cleaved from resin and purified to homogeneity by using RP-HPLC and mass spectrometry. Abbreviations: Aca, ε-aminocaproyl; HFIP, 1,1,1,3,3,3-hexafluoroisopropanol; TFE, 2,2,2-trifluoroethanol; TIPS, triisopropylsilane; TFA, trifluoroacetic acid; Aca, 6-amino caproic acid; t-BuOH, tert-butanol. Hyp, trans-4-hydroxy-l-proline; Thi, β-(2-thienyl)-l-alanine; NChg, N-cyclohexyl-glycine.
