Abstract
Epilepsy is one of the most common chronic disorders affecting individuals of all ages. A greater understanding of pathogenesis in epilepsy will likely provide the basis fundamental for development of new antiepileptic therapies that aim to prevent the epileptogenesis process or modify the progression of epilepsy in addition to treatment of epilepsy symptomatically. Therefore, several investigations have embarked on advancing knowledge of the mechanism underlying epileptogenesis, understanding in mechanism of pharmacoresistance and discovering antiepileptogenic or disease-modifying therapy. Animal models play a crucial and significant role in providing additional insight into mechanism of epileptogenesis. With the help of these models, epileptogenesis process has been demonstrated to be involved in various molecular and biological pathways or processes. Hence, this article will discuss the known and postulated mechanisms of epileptogenesis and challenges in using the animal models.
Key Words: Animal models, Epileptogenesis, Pathogenesis of epilepsy, Temporal lobe epilepsy
Introduction
Epilepsy is a neurological disorder characterized by recurrent and unpredictable interruptions of normal brain function, called epileptic seizure (1). It is one of the most common chronic disorders affecting around 50 million individuals of all ages worldwide (2). The median prevalence of life-time epilepsy for developed countries was 5.8 per 1,000 and for developing countries up to 15.4 per 1,000 (3). The median prevalence of active epilepsy despite treatment was 4.9 per 1,000 for developed countries and up to 12.7 per 1,000 for developing countries (3). This showed that prevalence of epilepsy is higher in developing countries compared to developed countries. In Asia, the median life-time prevalence is estimated at 6 per 1,000, which is lower than in developing countries in other areas of the world (4).
According to the causal etiology, epilepsy can be divided into 3 categories: idiopathic, acquired (symp-tomatic) and cryptogenic (presumed symptomatic) (5-7). Idiopathic epilepsy is epilepsy without underlying structural brain lesion or other neurologic signs or symptoms, which is presumed to be genetic and generally has onset during childhood. Acquired epilepsy is epileptic seizures as a result of one or more identifiable structural lesions of the brain. Cryptogenic epilepsy refers to the epilepsy that is believed to be symptomatic, with unidentifiedcause (5, 6). Among the epilepsy cases, approximately 40% have known etiology (8), including traumatic brain injury, ischemic stroke, intracerebral hemorrhage, central nervous system infections, brain tumors, several neurodegenerative diseases, and prolonged acute symptomatic seizures such as complex febrile seizures or status epilepticus (SE) (6,7,9).
On the other hand, the International League against Epilepsy has classified seizures into two major types namely generalized seizures which involve both hemispheres of brain and partial (focal) seizures which begin locally in one hemisphere of the brain (10). Majority of patients with epilepsy suffer from partial seizures (11). Temporal lobe epilepsy (TLE) is the most common and difficult-to-treat type of partial epilepsy (10, 11). This may be attributed to temporal lobe structures, typically the hippocampus, the amygdala and the piriform cortex which are most susceptible to epileptogenesis-triggering brain insult (12). Therefore, TLE is commonly investigated in order to understand the mechanism underlying epileptogenesis, antiepileptic pharmacoresistance and to discover antiepileptogenic or disease-modifying therapy. Animal models of epilepsy have been suggested to play important roles in these mechanistic studies (13, 14). Hence, this review article discusses the known and postulated mechanisms of epileptogenesis and challenges in using the animal models in epilepsy study. The databases that were used for literature searching included Science direct, PubMed and Wiley Online Library. The search keywords included pathogenesis of epilepsy, epileptogenesis, animal models, neurotransmission pathway, channelopathies, neurogenesis and rewiring pathway, inflammatory pathway, apoptotic pathway, gene regulation and other related keywords. Only articles published in English were reviewed.
Pathogenesis of epilepsy
Definition of epileptogenesis
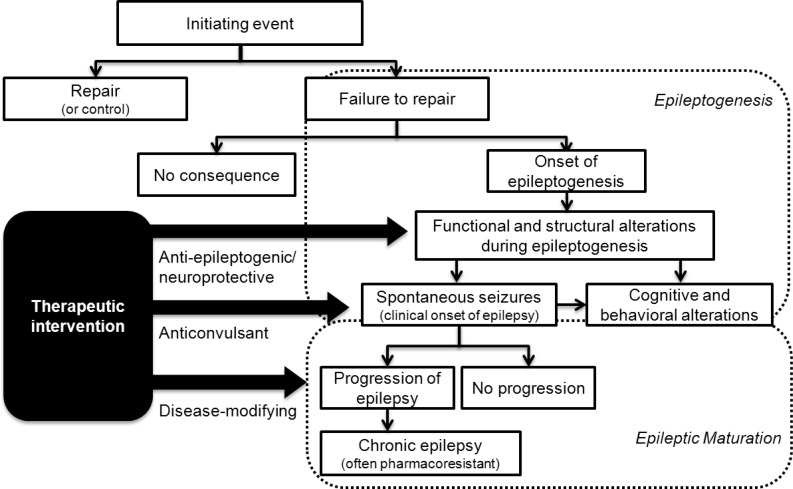
Currently, there is no universally accepted definition for epileptogenesis. The term epilepto-genesis is defined as a process that leads to the occurrence of the first spontaneous seizure and recurring epileptiform events after the brain insult (Figure 1) (15). Latency period refers to seizure-free or pre-epileptic periods between the brain insult and the occurrence of the first spontaneous seizure (15, 16). There is evidence that neurobiological changes that occur during the latency period continue to progress even after diagnosis of epilepsy and contribute to its progression (17, 18). Hence, the proposed definition for epileptogenesis includes processes that involve both development and progression of epilepsy (19). Nevertheless, recently, Sloviter Bumanglag proposed and defined secondary changes or progression process after epileptogenesis as ‘epileptic maturation’. They reviewed ‘epileptogenesis’ and ‘epileptic maturation’ as two distinct processes (15).
Figure 1.
Steps in the development and progression of epilepsy and possible therapeutic interventions (Adapted from (11) with permission)
Mechanism of epileptogenesis
Epileptogenesis can involve various biological pathways or processes, structural and functional changes. In general, it is unclear which mechanisms are required or necessary for the genesis of epilepsy. However, several experimental studies have provided some insights into the actual and postulated mechanisms of epileptogenesis.
Neurotransmission signaling pathway
Glutamate and γ-aminobutyric acid (GABA) are the two neurotransmitters that have been studied extensively in relation to epilepsy. Both glutam-atergic and GABAergic system play crucial roles in epileptic phenomena. It has been hypothesized that the neuronal hyperexcitability in epilepsy is due to imbalance between glutamate-mediated excitation and GABA-mediated inhibition (12). Glutamate is a main excitatory neurotrans-mitter in brain that is responsible for generating excitatory postsynaptic potentials by depolarizing the neurons (20, 21). Generally, glutamate receptors are classified into ionotropic (ligand-gated cation channels) receptors: α-amino- 3- hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA), N-methyl-D-aspartic acid (NMDA) and kainate, and metabotropic (G protein-coupled) receptors (20-22). Glutamatergic molecular mecha-nisms that are involved during initiation and progression of epilepsy include upregulation of glutamate receptors (23-25), elevation in extra-cellular glutamate concentration (26, 27), abnormalities in glutamatergic transporters (27, 28) and autoimmune mechanism (29). These mechan-isms contribute to excessive glutamatergic activity, which plays an important role in hyperexcitability and epilepsy (12). This phenomenon recorded as an interictal spike on electroencephalogram, known as ‘paroxysmal depolarizing shift’ is intracellularly associated with epileptic discharges in neurons (20, 30, 31). Paroxysmal depolarizing shift is associated with depolarization due to a giant excitatory synaptic potential with characteristic of burst discharge, which is dependent on activation of AMPA receptors as initial components and NMDA receptors as later components (30).
Conversely, GABA is recognized as the main inhibitory neurotransmitter, which generates inhibitory presynaptic potentials by hyperpolarizing the neurons (32, 33). GABAergic system has an important role in counter-balancing the neuronal excitation and therefore suppressing the epileptiform discharges (32). There are two types of GABA receptors that are involved in pathogenesis of epilepsy, namely GABAA and GABAB receptors. GABAA receptors (ligand-gated ion channels) mediate rapid inhibitory presynaptic potentials by increasing influx of chloride, and GABAB receptors (G-protein-coupled receptors) mediate slow inhibitory presynaptic potentials by increasing the potassium conductance and decreasing the calcium entry (33-35). It is hypothesized that reduction or loss of GABAergic inhibition may increase the probability of generating excitatory postsynaptic potentials and synchronizing burst discharges, and therefore induce epileptogenesis (12, 31). The GABAergic mechanisms that have been proposed include impairment of GABA release (36), changes in GABA receptors (37, 38), impairment of GABA synthesis (39, 40) and neuronal loss (41, 42).
Other neurotransmitters such as serotonin, noradrenaline and dopamine also play a role in epileptic mechanism. Serotonin, also known as 5-hydroxytryptamine is a monoamine neurotrans-mitter that is derived from amino acid tryptophan. There are several serotonin receptor subtypes expressed in the central nervous system, such as 5-HT1A, 5-HT2C and 5-HT7. Receptors are present on cortical and/ or hippocampal neurons (43, 44). Experimental data from animal models and humans reveal that serotonergic neurotransmission is significantly involved in pathogenesis of epilepsy: depletion of brain serotonin in genetically epilepsy-prone rat model of audiogenic seizures (45, 46); lower seizure thresholds in mutants mice lacking 5-HT1A or 5-HT2C receptor subtype (47, 48); decrease in 5-HT1A receptor binding in epileptogenic zone of TLE patients (49). The serotonergic system in modulating neuronal excitability has been complicated by diversity of serotonin receptor subtypes. Generally, neuronal excitability can be reduced during hyperpolarization of glutamatergic neurons by 5-HT1A receptors, depolarization of GABAergic neurons by 5-HT2C receptors and inhibition of 5-HT3 and 5-HT7 receptors (44).
Noradrenaline is a catecholamine produced from dopamine, which is released either as a hormone from adrenal medulla or as a neurotransmitter in the central and sympathetic nervous systems from noradrenergic neurons (50). Multiple studies demonstrate that endogenous noradrenaline has an anticonvulsant role in epilepsy. These include noradrenaline depletion increased susceptibility to seizure induction (51, 52) and noradrenaline loss increased neuronal damage in various limbic regions of rats after seizure induction (53). It is postulated that the protective effect of noradrenaline is attributed to counteraction of an epileptic circuit formation and modification in the epilepsy-induced neuronal changes (54).
Another catecholamine neurotransmitter, dopam-ine, exerts an ambiguous and complex pathway in pathogenesis of epilepsy. Researchers have found that dopaminergic pathway is associated with pathophysiology of two idiopathic epilepsies, i.e. autosomal dominant nocturnal frontal lobe epilepsy with significant reduction in dopamine D1 receptor binding (55) and juvenile myoclonic epilepsy with decrease in binding potential to the dopamine transporter (56). This is consistent with the hypothesis that decrease in inhibitory dopaminergic activity predisposes to hyperexcitability and epilepsy (57). However, activation of different dopamine receptor families (D1 and D2) may produce diverging effects on neuronal excitability, in which D1 receptor has proconvulsant effect (58) and D2 receptor has anticonvulsant effect (58-60). A recent study in TLE patients found that there was a decrease in dopamine D2/D3 receptor binding in epileptogenic zone of these patients (61). These evidences show that dopamine might play a specific role in modulating seizures.
Molecular and genetic mechanisms: Ion channels and receptors
Recent advances in genetics and molecular biology have demonstrated that several epilepsy syndromes are attributed to mutations in genes encoding ion channel proteins that lead to hyperexcitability of neurons (31, 62, 63). Channelopathy is a term used to describe ion channel dysfunction or defect (63). Ion channels are pore-forming proteins along the lipid membrane of cells that allow movement of selected ions across cell membranes to maintain negative resting membrane potential inside the cells (63, 64). There are two types of ion channels, which are voltage-gated channels controlled by changes in membrane potential and ligand-gated channels that are activated by ligand binding such as GABA and acetylcholine neurotransmitters (63). Ion channels are involved in generating electric currents via ion charges. Generally, cation channels mainly generate action potentials and contribute to neuronal excitability, in contrast, anion channels are involved in inhibitory mechanism for neuronal excitatory process (65). Hence, it is hypothesized that in imbalance of ion charges due to channelopathies, either anion or cation channel can induce epileptogenesis (65).
Channelopathies are key factors of pathogenesis in human epilepsy, predominantly in idiopathic epilepsy (66, 67). Mutations in genes expressing channels of potassium, sodium, chloride, calcium and receptors of acetylcholine and GABA have been reported in idiopathic epilepsy (Table 1) (63, 68). In addition, channelopathies can also be the pathogenesis in acquired epilepsy due to secondary changes in ion channels via transcriptional and post-translational mechanisms (66, 67).
Table 1.
| Epilepsy phenotype | Channel (Gene involved) |
|||||
|---|---|---|---|---|---|---|
| Sodium | Potassium | Chloride | Calcium | GABA | Acetylcholine | |
| Autosomal dominant nocturnal frontal lobe epilepsy | CHRNA4, CHRNB2 | |||||
| Benign familial neonatal infantile seizures | SCN2A | |||||
| Benign familial neonatal seizures | KCNQ2,KCNQ3 | |||||
| Childhood absence epilepsy | CLCN2 | CACNA1H | GABRG2 | |||
| Epilepsy with grand mal seizures on awakening | CLCN2 | |||||
| Episodic ataxia type 1 | KCNA1 | |||||
| Episodic ataxia type 2 | CACNA1A, CACNB4 | |||||
| Familial hemiplegic migraine | CACNA1A | |||||
| Febrile seizures | GABRG2 | |||||
| Generalized epilepsy with febrile seizures plus | SCN1A, SCN2A, SCN1B | GABRG2 | ||||
| Generalized epilepsy with paroxysmal dyskinesia | KCNMA1 | |||||
| Infantile spasms | SCN1A | |||||
| Intractable childhood epilepsy with generalized tonic-clonic seizures | SCN1A | |||||
| Juvenile absence epilepsy | CLCN2 | |||||
| Juvenile myoclonic epilepsy | CLCN2 | GABRA1,GABRD | ||||
| Myokymia | KCNQ2 | |||||
| Severe myoclonic epilepsy of infancy | SCN1A | GABRG2 | ||||
| Spinocerebellar ataxia type 6 | CACNA1A | |||||
On the other hand, recent studies have found that channelopathy involves the hyperpolarization-activated cyclic nucleotide gated (HCN) channels that may contribute to TLE (69) and absence seizure (70). HCN channels are voltage-gated ion channels that conduct the hyperpolarization-activated cationic current, Ih, which regulate resting membrane potential of neurons (71, 72). HCN channels are activated by membrane hyperpolarization and lead to inhibitory effects of Ih on neuronal excitability (71, 72). Experimental animal model studies have shown that down regulation of HCN channels and loss of channel expression subsequently cause a reduction in Ih density, and ultimately contribute to neuronal hyperexcitability (69, 73). Hence, HCN channelo-pathy may play a role in epileptogenesis.
Neurogenesis and rewiring pathway: Structural, neurochemical and cellular changes
Aberrant hippocampal neurogenesis, a process of new neurongeneration, and new circuit creation has been proposed as another important pathogenesis in epilepsy (74, 75). Various changes include structural, neurochemical and cellular changes which may occur following acute seizures in patients with brain insults (76).
Multiple structural alterations in the hippocampus could occur after acute seizures, including degeneration of dentate hilar neurons and CA1 – CA3 pyramidal neurons, aberrant sprouting and synaptogenesis of mossy fibers and loss of inhibitory GABAergic interneurons (76). Mossy fiber sprouting involves synaptic reorganization of mossy fibers, which are axons of dentate granule cells for forming new synaptic contacts with an abnormal location, the inner third molecular layer of dentate gyrus or the supragranular area (77, 78). This structural reorga-nization would be attributable to synapse elimination due to neuronal death of mossy cells, which are principal excitatory neurons in dentate hilus that normally project to the supragranular area (78, 79). As a consequence of axon sprouting and synaptogenesis in mossy fiber pathway, the new neuronal circuitry forms a recurrent excitatory circuit in dentate granule cells that is expected to increase excitatory drive and eventually promote epileptogenesis (78, 79). Another proposed hypo-thesis of structural reorganization is the functional disconnection of dormant basket (inhibitory) cells with excitatory neurons. This hypothesis implies that GABAergic interneurons (basket cells) survive after an epileptogenic injury, but they remain in dormant state and are unable to provide feedback inhibition to granule cells due to seizure-induced death of major excitatory neurons) mossy cells( that results in reducing excitatory drive on these basket cells (80, 81). Dormancy of basket cells leads to loss of inhibition, possibly contributing to epileptogenesis.
Apart from structural changes, acute seizures can also up regulate several neurotropic factors and other proteins in the hippocampus. These include nerve growth factor (82, 83), brain-derived neuro-trophic factor (82, 83), fibroblast growth factor-2 (84), vascular endothelial growth factor (VEGF) (85) and sonic hedgehog (86). The neurotrophins, i.e. nerve growth factor, brain-derived neurotrophic factor and fibroblast growth factor-2, have a role in neuronal survival, differentiation, growth, synaptic plasticity and excitability (32). VEGF induces angiogenesis, but increase in VEGF could contribute to blood brain barrier disruption and inflammation in brain (85). On the other hand, sonic hedgehog is a secreted protein that regulates the proliferation and survival of neuronal and glial precursors (86). Up-regulation of these proteins might contribute to neurogenesis in hippocampus (76).
Cellular changes are also involved in the hippo-campus following acute seizures. These changes include increase of neurogenesis, abnormal migra-tion of newly born granule cells into dentate hilus and dentate molecular layer, and occurrence of hilar basal dendrites in newly added granule cells (74-76). All these changes might form an aberrant circuitry that contributes to generation of epileptiform activity by creating excitatory loops and thus enhance seizure initiation and propagation.
Immunological and inflammatory pathway
Cytokines are polypeptide mediators that are associated with activation of immune system and inflammatory reactions (87). Recent studies in animal models have shown that inflammatory cytokines are involved in the pathogenesis of epilepsy. The inflammatory cytokines such as interleukin (IL)-1β, IL-6 and tumor necrosis factor-α have been shown to be up-regulated and over-expressed in brain regions involved in generating and propagating epileptic activity (88-90). Glial cells, particularly microglia and astrocytes, the non-neuronal cell components in central nervous system, are known to be sources for the inflammatory cytokines in the epileptic tissues (90-92). Hence, glial cells play a role in regulating immune or inflammatory response during epileptogenesis. The release of inflammatory cytokines in microglia and astrocytes is usually followed by a cascade of down-stream inflammatory events which can recruit neurons and activate adaptive immune system (93, 94).
However, there is concern on how the activated inflammatory pathway contributes to pathogenesis of epilepsy. It has been revealed that these inflammatory cytokines have deleterious effects on neurons via alteration of neuronal excitability, production of toxic mediators and increase impermeability of blood-brain barrier (BBB) (91,94). IL-1β can induce the activation of NMDA receptor, thus enhancing NMDA-mediated ion calcium influx into neurons and ultimately promoting neuronal hyperexcitability (95, 96). Similar to IL-1β, tumor necrosis factor-α can also induce neuronal excitability via up-regulation of AMPA receptors which favors the ion calcium influx into neuron and down-regulation of GABA receptors in which the inhibitory synapse strength decreases(97). Apart from excitotoxic effects, inflammatory cytokines may contribute to apoptotic neuronal death, which is likely due to production of neurotoxic mediators and NMDA- and AMPA-mediated glutamatergic excitotoxicity (95). Besides, inflammation reactions may alter the BBB permeability (90, 91). The BBB disruption may induce epileptogenesis by uptake of serum albumin into astrocytes via binding to transforming growth factor-β receptor and triggering subsequent events that contribute to neuronal hyperexcitability and eventually epileptiform activity (98, 99).
In addition, Febene and colleagues (100) showed that inflammatory cell adhesion has a role in seizure pathogenesis. They showed that expression of vascular cell adhesion molecules is elevated and leukocyte adhesion to endothelial cells is enhanced in cerebral blood vessels, which is mediated by leukocyte mucin P-selectin glycoprotein ligand-1 and leukocyte integrins after pilocarpine-induced seizure (100). Consequently, this results in a cascade of events including increased leukocyte extravasation, cerebral inflammation, BBB leakage, enhance neuro-nal excitatory and ultimately epileptogenesis (100).
Apoptotic pathway
Apoptosis is a programmed cell death process during normal growth and development in multicellular organisms for maintaining cell homeostasis (101). Experimental and clinical data have shown that significant neuronal cell loss occurs after brain insults and apoptotic pathway may be involved in this cell loss in addition to other mechanisms such as excitatory glutamate-mediated toxicity (102-105). There are two major families of genes that regulate the apoptosis pathway in mammals: caspases and Bcl-2 family proteins (106). Caspases are a family of cysteine proteases and mainly function as apoptotic initiator (caspases 2, 8, 9, 10) or executioner (caspases 3, 6, 7) in apoptosis process (102). Bcl-2 family proteins are important regulators of the apoptosis process in cellular life and death decision. This characteristic is attributed to its anti-apoptotic (e.g. Bcl-2, Bcl-XL, Bcl-W and Mcl-1) and pro-apoptotic members (e.g. Bax, Bak, Bad, Bid and other BH-3 only proteins) (102, 107). Apart from caspases and Bcl-2, other proteins such as p53, tumor necrosis factor, Fas ligand and nuclear factor-κB are also essential in regulating the cell death mechanisms (102, 108).
In experimental models of epilepsy and epilepto-genesis, researchers have revealed that executioner caspase-3 and -6 are activated and actively expressed in the hippocampus (103, 109, 110). Be-sides, Bcl-2 family proteins such as Bax and Bcl-2 are also involved in pathogenesis of human temporal lobe epilepsy models (102, 106, 111). These eviden-ces suggested that apoptotic pathway may play a role in the pathogenesis of epilepsy.
Gene and protein regulation
Several studies have demonstrated that alteration in gene expression is triggered after brain insults and have proposed that this might be regulated by transcription factors. Cellular immediate early genes or inducible transcription factors such as members of the Jun family (c-jun, junB, junD) and the Fos family (c-fos, fosB and fos-related antigenesfra-1 and fra-2) are believed to be involved in pathogenesis of seizures (112). Both of these gene families encode transcription factors c-fos and c-jun, which are the major components of the transcription factor activator protein-1 (113). Early up-regulation and expression of c-fos and c-jun mRNA have been demonstrated in hippocampal neurons during experimental seizures (114, 115) or cerebral ischemia (116). The specific roles of IEGs immediate early genes in epileptogenesis have not been elucidated. Generally, in central nervous system, transcription factors of Fos and Jun families are involved in gene transcription, cell proliferation, regeneration and cell death (112, 113, 117, 118). The expression of immediate early genes may partly form the biological cascade that induces apoptotic cell death of neurons (117).
Another transcription factor, inducible cyclic adenoside monophosphate (cAMP) early repressor (ICER) also plays a role in epileptogenesis. ICER is a group of proteins produced from the cAMP-responsive element modulator (CREM) gene and ICER messenger RNAs that are transcribed by an internal promoter of the CREM (119). ICER serves as an endogenous repressor of cAMP-responsive element (CRE)-mediated gene transcription (120, 121). With this characteristic, ICER plays a role as important transcriptional regulator of neuronal plasticity and apoptosis in nervous system by repressing CRE-mediated gene transcription and antagonizing activity of cAMP-responsive element binding protein (CREB) transcription factor. CREB is an activator for CRE transcription that is crucial for neuronal survival (120, 121). Up-regulation of ICER expression in neurons after excitotoxic stimuli and over-expression of ICER in cultured neurons have shown apoptotic effect (122). Controversially, recent studies have suggested that high expression of ICER suppresses the kindling process in experimental animal models (123, 124). Therefore, the mechanism of ICER in epileptogenesis still remains unclear.
Changes of neuropeptides expression in hippo-campus following seizure have been observed in experimental studies. These neuropeptides include neuropeptide Y (NPY), somatostatin, cholecysto-kinin, neurokinin B, galanin, thyrotropin-releasing hormone and cortistatin (125-127). In hippocampus, NPY is co-localized in GABAergic interneurons and hence, it has inhibitory actions on neuronal excitation (128, 129). Over-expression of NPY and its mRNA in hippocampus (125,126,130) and aberrant expression of NPY in hippocampal granule cells and mossy fibers that normally do not contain this peptide (130, 131) have been reported in experi-mental seizure studies. It has been suggested that these findings represent endogenous adaptive mechanism to counteract the hyperexcitability state during seizure stimulation (129). Collaterally, this concept is supported by studies that showNPY gene therapy in animal models reduced the spontaneous seizure and delayed the progression of seizure (132, 133).
Another neuropeptide, galanin also has been demonstrated to be involved in modulating seizure activity (134). Galanin is widely distributed throughout the central nervous system and is involved in various brain functions (135). It is well-known as a universal neurotransmitter inhibitor, by inhibiting the release of neurotransmitters including glutamate, acetylcholine and noradrenaline (135). In other words, it serves as a seizure modulator by restoring the balance between glutamatergic excitation and galaninergic inhibition in dentate gyrus of hippocampus (135) through the activation of galanin receptors GalR1 and GalR2 (136, 137). Depletion of stored galanin in dentate hilus has been observed after exposure to seizure stimuli, interestingly the galanin expression reappeared and increased a few hours after the stimulation (134). In addition, studies on over-expression of galanin in animal models have shown that galanin played a significant role in decreasing the susceptibility of seizure during seizure induction (138). The available evidences supported the role of gene and protein regulation in epileptogenesis.
Animal models for epileptogenesis
A variety of animal models have been developed to study epilepsy and epileptic seizures. Each animal model demonstrates different types of epilepsy (Table 2) (14, 44).However, each model is unique for a specific study purpose (14, 139). The objective of each experiment is essential for selection of a suitable animal model. For example, mechanism of epileptogenesis can be explored using several models such as kindling, post-SE with spontaneous recurrent seizure, traumatic brain injury-induced epilepsy, stroke-induced epilepsy and febrile seizure models (8, 140). In addition, genetic animal models of generalized epilepsy, such as tottering mice with spontaneous recurrent seizures and genetically epilepsy prone rats with reflex seizures can also be employed (8, 141). Nevertheless, not all these models are suitable for the testing antiepileptogenic or disease-modifying therapies (139). The National Institutes of Health/National Institute of Neurological Disorders and Stroke Workshop recommended only two models as useful for antiepileptogenic treatment discovery, which are kindling and post-SE models with spontaneous recurrent seizure (139).
Table 2.
| Type of epilepsy | Animal models* |
|---|---|
| Partial seizures | |
| Acute seizures | Electrical stimulation, e.g. 6-Hz |
| Chronic seizures | Electrical or chemical kindling Topical chemoconvulsants, which block inhibition, e.g. penicillin, bicuculline, picrotoxin, pentylentetrazol, strychnine Topical chemoconvulsants, which enhance excitation, e.g. carbachol, kainate Freeze lesion or partially isolated cortical slab Implanted metals, e.g. Al2O3, cobalt Experimental febrile seizures Post-traumatic epilepsy (PTE) induced by lateral fluid percussion brain injury Hippocampal sclerosis model, e.g. kainic acid, pilocarpine, post-status epilepticus models Focal dysplasia model, e.g. neonatal freeze, prenatal radiation, methylazoxymethanol |
| Post-status epilepticus models with spontaneous recurrent seizures | Electrical status epilepticus induction, e.g. perforanth path, basolateral amygdala Chemical status epilepticus induction, e.g. pilocarpine, kainate |
| Generalized seizures | |
| Generalized tonic-clonic seizures | Electrical stimulation, e.g. maximal electroshock Chemoconvulsants, e.g. pilocarpine, kainate, penthylenetetrazol, bicuculline, picrotoxin, flurothyl Genetic models, e.g. genetically epilepsy-prone rats, Mongolian gerbil, DBA/2J mice, photosensitive baboons, knockout mice |
| Absence seizure | Chemoconvulsants, e.g. low dose penthylenetetrazol, gamma-hydroxybutyrate Genetic models, e.g. genetic absence rats from Strasburg, Wistar Albino Glaxo/Rijswijk, tottering mice, stargazer mice, lethargic mice, slow-wave epilepsy mice, mocha mice, ducky mice |
*Common animals used are rats and mice
Kindling is a model of chronic seizures which involves progressive intensification of brain excitability by repeated excitatory stimuli (electrical or chemical) that ultimately induce seizure disorder (14, 142). Electrical kindling usually stimulates a specific brain region, such as amygdala, hippocampus or other brain regions, via chronically implanted depth electrodes (32, 142, 143). Chemical kindling, such as pentylenetetrazoleis ultilized in some studies, but this method has been much less utilized than electrical kindling (144, 145). Pentylenetetrazole kindling involves repeated injection of pentylenetetrazole to cause gradual seizure development as a result of which a significant neuronal loss in hippocampus CA1 and CA3 structures have been observed (144). Kindling model is widely used as a model of TLE because the fully kindled seizures resemble the complex partial seizures and secondarily generalized seizures (32, 143).
Post-SE model with spontaneous recurrent seizure also comprises electrically induced models (e.g. electrical stimulation of hippocampus via perforant path, angular bundle or CA3 of ventral hippocampus and lateral or basolateral amygdala) and chemically induced models (e.g. pilocarpine or kainate) (13, 14, 146, 147). The spontaneous recurrent, partial and secondarily generalized seizures, damage of hippocampal and extrahippocampal, and alterations of behavior and cognition produced by the pilocar-pine- and kainate-induced models resemble clinical characteristics of TLE, hence, both are considered as representative models for TLE (32, 147).
Challenges in animal models
Undoubtedly, animal models have provided useful information in addressing critical research issues, including mechanism of epileptogenesis, which are impossible to study in humans due to ethical concerns. However, what is the degree that these models reflect the actual condition in humans with epilepsy? The actual mechanisms underlying epilepsy may be more complicated. For example, in TLE studies, post-SE models involved an acute triggering SE process that was frequently followed by a latency period with subsequent development of spontaneous motor seizure (148, 149) and hippocampal lesions similar to that showed in TLE patients (150-152). Nevertheless, these models cause neuronal loss not only in hippocampus and amygdale of the limbic area, but also extralimbic regions such as thalamus, hypothalamus and certain areas of celebral cortex, which are usually not involved in human TLE (147, 151). Hence, there is a need to develop new animal models that are able to reproduce unique characteristics of epilepsy as faithfully as possible and therefore, enhance the extrapolation of animal data to human condition.
The animal models have a different etiologic process compared to status epilepticus in human, which is usually associated with traumatic brain injury and ischemic stroke (6, 9). Hence, it is difficult to justify whether animal models are able to ideally or adequately model human epileptogenesis process. Generally, it is believed that common mechanisms might underlie human and animal epileptogenesis (152). Another challenge for the animal model is related to various subtypes of either generalized or partial seizures in human epilepsy such as generalized myoclonic seizure, generalized atonic seizure and absence seizure (10). However, animal models usually manifest generalized tonic-clonic and limbic seizures only (140), which represent sub-population of patients with these types of epilepsy. Hence, this can limit current understanding on epileptogenesis in other epilepsy phenotypes.
Application of the animal models in understanding other epileptic related conditions such as cognitive and behavioral changes could be improved. Many patients with TLE suffer from cognitive and behavioral alterations, such as depression, anxiety, psychosis and memory loss, which may be associated with morphologic and functional alterations in the temporal lobe (153). These behavioral alterations are difficult to evaluate directly in the current available models. Different tests have been designed to complement the assessment of behavioral alteration in animals, for instance, test for anxiety-related behavior (e.g. light/dark box test, elevated plus-maze and open-field tests), Irwin test for evaluating physiologic reflexes and behavioral abnormalities, and test for depression-like behavior (e.g. forced swimming test and tail suspension test) (154). These tests are useful for predicting the anxiety level in mice and provide insight into association between epilepsy and behavioral alterations.
There is a substantial degree of variability among individual animals in response to epileptogenic or convulsive stimuli in a specific model. In rodents for example, strain, gender and age factors affected the response to epileptogenic stimuli (151, 155, 156). Immature rats were more susceptible to status epilepticus than were adult rats, but immature hippocampi exhibit markedly less hippocampal damage and changes compared to adults after treatment with kainate or repeated kindling (157). Apart from age, different strains and genders may have different responses to epileptogenic stimuli. Female Sprague-Dawley rats were more sensitive to basolateral amygdala stimulation compared to Wistar rats in terms of status epilepticus induction and development of epilepsy after SE (156). Besides diversity in susceptibility to stimuli, strain factor also influences the patterns of hippocampal damage and neurodegeneration in affected brain areas (158). Such variability in animals may give results that cannot be reproduced from one laboratory to another and consequently, increases the difficulties in generalizing the findings in a specific model (159).
Currently, these animal models still remain valuable research tools in view of presence of an intact central nervous system, which provides an opportunity for studying the mechanism underlying epileptogenesis. There is no general agreement about which animal model may be most appropriate and relevant to human condition and none of the available models have been clinically validated (7). For a model to be validated, it should be highly predictive of clinical response, which is usually complicated by many forms of epilepsy with different pathophysiologies (141). Hence, to improve current animal models, the limitations and confounding factors should be taken into consideration in developing a novel animal model.
Conclusion
A greater understanding of pathogenesis of epilepsy will likely provide the basis fundamental for development of new antiepileptic therapies that aim to prevent the epileptogenesis process, or modify the progression of epilepsy in addition to treatment of epilepsy symptomatically. Animal models play a crucial and significant role in providing additional insight into mechanism of epileptogenesis, predo-minantly kindling and post-SE with spontaneous recurrent seizure models (7,140). With the help of these models, epileptogenesis process has been demonstrated to be involved in various molecular and biological pathways or processes. These include neurotransmission signaling pathway, molecular and genetic mechanism, neurogenesis and rewiring pathway, immunology and inflammatory pathway, apoptotic pathway, gene and protein regulation and other mechanisms that are not discussed in this article. However, it still remains unclear which mechanisms are required or necessary for genesis of epilepsy in human. Besides, there are some challenges that arise from the use of animal models in experimental studies, such as degree of representation from animal models to epileptic human models, different etiologies of seizure induction between animal models and human models, difficulty of evaluating behavioral alteration in animal models and variability among individual animals. In order to overcome these challenges, currently available animal models should be used with caution, validated and all the limitations and confounding factors should be taken into consideration. This could provide a more accurate picture of the epileptogenesis process and ultimately, contribute to development of new antiepileptogenic or disease-modifying therapies, which provide new hope for epileptic patients.
References
- 1.Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Epilepsy: Factsheet. c2009. [cited 2012 Oct 9]. Available at: http://www.who.int/mediacentre/factsheets/fs999/en/index.html.
- 3.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mac TL, Tran DS, Quet F, Odermatt P, Preux PM, Tan CT. Epidemiology, aetiology and clinical management of epilepsy in Asia: A systematic review. Lancet Neurol. 2007;6:533–543. doi: 10.1016/S1474-4422(07)70127-8. [DOI] [PubMed] [Google Scholar]
- 5.Engel JJ. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of ILAE Task Force on classification and Terminology. Epilepsia . 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 6.Commission on Classification and Terminology of the International League Against Epilepsy (ILAE) Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia . 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 7.Loscher W, Brandt C. Prevention or Modification of Epileptogenesis after Brain Insults: Experimental Approaches and Translational Research. Pharmacol Rev. 2010;62:668–700. doi: 10.1124/pr.110.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee PN, Filippi D, Hauser WA. The descriptive epidemiology of epilepsy – A review. Epilepsy Res. 2009;85:31–45. doi: 10.1016/j.eplepsyres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annegers JF, Rocca WA, Hauser WA. Causes of epilepsy: Contributions of the Rochester epidemiology project. Mayo Clin Proc. 1996;71:570–575. doi: 10.4065/71.6.570. [DOI] [PubMed] [Google Scholar]
- 10.Engel JJ. Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26:141–150. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- 11.Loscher W, Gernert M, Heinemann U. Cell and gene therapies in epilepsy-Promising avenues or blind alleys? . Trends Neurosci. 2008;31:62–73. doi: 10.1016/j.tins.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Aroniadou-Anderjaska V, Fristch B, Qashu F, Braga MF. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res. 2008;78:102–116. doi: 10.1016/j.eplepsyres.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002;50:105–123. doi: 10.1016/s0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- 14.Loscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Sloviter RS, Bumanglag AV. Defining “epileptogenesis” and identifying “antiepileptogenic targets” in animal models of acquired temporal lobe epilepsy is not as simple as it might seem. Neuropharmacology. 2012;69:3–15. doi: 10.1016/j.neuropharm.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitkanen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. 186. 2011;10 doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 17.Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1:173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- 18.William PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, et al. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitkanen A, Bolkvadzea T, Immonenc R. Anti-epileptogenesis in rodent post-traumatic epilepsy models. Neurosci Lett. 2011;497:163–171. doi: 10.1016/j.neulet.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 20.Meldrum BS, Akbar MT, Chapman AG. Glutamate receptors and transporters in genetic and acquired models of epilepsy. Epilepsy Res. 1999;36:189–204. doi: 10.1016/s0920-1211(99)00051-0. [DOI] [PubMed] [Google Scholar]
- 21.Voglis G, Tavernarakis N. The role of synaptic ion channels in synaptic plasticity. EMBO Rep. 2006;7:1104–1110. doi: 10.1038/sj.embor.7400830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology. 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- 23.Seifert G, Schroder W, Hinterkeuser S, Schumacher T, Schramm J, Steinhauser C. Changes in flip/flop splicing of astroglial AMPA receptors in human temporal lobe epilepsy. Epilepsia. 2002;43:162–167. doi: 10.1046/j.1528-1157.43.s.5.10.x. [DOI] [PubMed] [Google Scholar]
- 24.Debanne D, Thompson SM, Gahwiler BH. A brief period of epileptiform activity strengthens excitatory synapses in the rat hippocampus in vitro. Epilepsia. 2006;47:247–256. doi: 10.1111/j.1528-1167.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 25.Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in rective astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12:2333–2344. doi: 10.1046/j.1460-9568.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- 26.DeLorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: The calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol Ther. 2005;105:229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi J, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Touret M, Parrot S, Denoroy L, Berlin MF, Didier-Bazes M. Glutamatergic alterations in the cortex of genetic absence epilepsy rats. BMC Neurosci. 2007;8:69–75. doi: 10.1186/1471-2202-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bien CG, Granata T, Antozzi C, Cross JH, Dulac O, Kurthen M, et al. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: A European consensus statement. Brain. 2005;128:454–471. doi: 10.1093/brain/awh415. [DOI] [PubMed] [Google Scholar]
- 30.Chapman AG. Glutamate and epilepsy. J Nutr. 2000;130:1043S–1045S. doi: 10.1093/jn/130.4.1043S. [DOI] [PubMed] [Google Scholar]
- 31.Acharya JN. Recent advances in epileptogenesis. Curr Sci. 2002;82:679–688. [Google Scholar]
- 32.Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42:8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- 34.Olsen RW, Avoli M. GABA and epileptogenesis. Epilepsia . 1997;38:399–407. doi: 10.1111/j.1528-1157.1997.tb01728.x. [DOI] [PubMed] [Google Scholar]
- 35.Meldrum BS. GABAergic mechanisms in the pathogenesis and treatment of epilepsy. Br J Clin Pharm. 1989;27:3S–11S. doi: 10.1111/j.1365-2125.1989.tb03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buzzi A, Chikhladze M, Falcicchia C, Paradiso B, Lanza G, Soukupova M, et al. Loss of cortical GABA terminals in Unverricht-Lundborg disease. Neurobiol Dis . 2012;47:216–224. doi: 10.1016/j.nbd.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Schwarzer C, Tsunashima K, Wanzenbock C, Fuchs K, Sieghart W, Sperk G. GABAA receptor subunits in the rat hippocampus II: Altered distribution in kainic acid-induced temporal lobe epilepsy. Neuroscience . 1997;80:1001–1017. doi: 10.1016/s0306-4522(97)00145-0. [DOI] [PubMed] [Google Scholar]
- 38.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med . 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 39.Dinkel K, Meinck HM, Jury KM, Karges W, Richter W. Inhibition of γ-aminobutyric acid synthesis by glutamic acid decarboxylase autoantibodies in stiff-man syndrome. Ann Neurol . 1998;44:194–201. doi: 10.1002/ana.410440209. [DOI] [PubMed] [Google Scholar]
- 40.Butler MH, Solimena M, Dirkx R, Hayday A, De Camilli P. Identification of a dominant epitope of glutamic acid decarboxylase (GAD-65) recognized by autoantibodies in stiff-man syndrome. J Exp Med . 1993;178:2097–2106. doi: 10.1084/jem.178.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci . 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knopp A, Frahm C, Fidzinski P, Witte OW, Behr J. Loss of GABAergic neurons in the subiculum and its functional implications in temporal lobe epilepsy. Brain . 2008;131:1516–1527. doi: 10.1093/brain/awn095. [DOI] [PubMed] [Google Scholar]
- 43.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology . 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 44.Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. J Neurochem . 2007;100:857–873. doi: 10.1111/j.1471-4159.2006.04277.x. [DOI] [PubMed] [Google Scholar]
- 45.Dailey JW, Yan Q, Adams-Curtis LE, Ryu JR, Ko KH, Mishra PK, et al. Neurochemical correlates of antiepileptic drugs in the genetically epilepsy-prone rat (GEPR) Life Sci . 1996;58:259 –266. doi: 10.1016/0024-3205(95)02286-4. [DOI] [PubMed] [Google Scholar]
- 46.Dailey JW, Mishra PK, Ko KH, Penny JE, Jobe PC. Serotonergic abnormalities in central nervous system of seizure-naïve genetically epilepsy-prone rats. Life Sci . 1992;50:319–326. doi: 10.1016/0024-3205(92)90340-u. [DOI] [PubMed] [Google Scholar]
- 47.Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin 1A receptors. Proc Natl Acad Sci USA . 2000;97:14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Applegate CD, Tecott LH. Global increases in seizure susceptibility in mice lacking 5-HT2C receptors: a behavioral analysis. Exp Neurol . 1998;154:522–530. doi: 10.1006/exnr.1998.6901. [DOI] [PubMed] [Google Scholar]
- 49.Merlet I, Ryvlin P, Costes N, Dufournel D, Isnard J, Failenot I, et al. Statistical parametric mapping of 5-HT1A receptor binding in temporal lobe epilepsy with hippocampal ictal onset on intracranial EEG. Neuroimage . 2004;22:886–896. doi: 10.1016/j.neuroimage.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Tully K, Bolshakov VY. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. 2010 Mol Brain ;3:15. doi: 10.1186/1756-6606-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corcoran ME, Mason ST. Role of forebrain catecholamines in amygdaloid kindling. Brain Res . 1980;190:473–484. doi: 10.1016/0006-8993(80)90289-9. [DOI] [PubMed] [Google Scholar]
- 52.Szot P, Weinshenker D, White SS, Robbins CA, Rust NC, Schwartzkroin PA, et al. Norepinephrine-deficient mice have increased susceptibility to seizure-inducing stimuli. J Neurosci . 1999;19:10985–10992. doi: 10.1523/JNEUROSCI.19-24-10985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giorgi FS, Ferrucci M, Lazzeri G, Pizzanelli C, Lenzi P, Alessandrl MG, et al. A damage to locus coeruleus neurons converts sporadic seizures into self-sustaining limbic status epilepticus. Eur J Neurosci . 2003;17:2593–2601. doi: 10.1046/j.1460-9568.2003.02692.x. [DOI] [PubMed] [Google Scholar]
- 54.Giorgi FS, Pizzanelli C, Biagioni F, Murri L, Fornai F. The role of norepinephrine in epilepsy: from the bench to the bedside. Neurosci Biobehav Rev. 2004;28:507–524. doi: 10.1016/j.neubiorev.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Fedi M, Berkovic SF, Scheffer IE, O’Keefe G, Marini C, Mulligan R, et al. Reduced striatal D1 receptor binding in autosomal dominant nocturnal frontal lobe epilepsy. Neurology . 2008;71:795–798. doi: 10.1212/01.wnl.0000316192.52731.77. [DOI] [PubMed] [Google Scholar]
- 56.Ciumas C, Robins Wahlin TB, Jucaite A, Lindstrom P, Halldin C, Savic I. Reduced dopamine transporter binding in patients with juvenile myoclonic epilepsy. Neurology . 2008;71:788–794. doi: 10.1212/01.wnl.0000316120.70504.d5. [DOI] [PubMed] [Google Scholar]
- 57.Benardo LS, Prince DA. Dopamine modulates a Ca2+-activated potassium conductance in mammalian hippocampal pyramidal cells. Nature. 1982;297:76–79. doi: 10.1038/297076a0. [DOI] [PubMed] [Google Scholar]
- 58.Barone P, Palma V, DeBartolomeis A, Tedeschi E, Muscettola G, Campanella G. Dopamine D1 and D2 receptors mediate opposite functions in seizures induced by lithium–pilocarpine. Eur J Pharmacol . 1991;195:157–162. doi: 10.1016/0014-2999(91)90394-6. [DOI] [PubMed] [Google Scholar]
- 59.Bozzi Y, Vallone D, Borrelli E. Neuroprotective role of dopamine against hippocampal cell death. J Neurosci. 2000;20:8643–8649. doi: 10.1523/JNEUROSCI.20-22-08643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clinckers R, Smolders I, Meurs A, Ebinger G, Michotte Y. Anticonvulsant action of hippocampal dopamine and serotonin is independently mediated by D2 and 5-HT1A receptors. J Neurochem . 2004;89:834–843. doi: 10.1111/j.1471-4159.2004.02355.x. [DOI] [PubMed] [Google Scholar]
- 61.Werhahn KJ, Landvogt C, Klimpe S, Buchholz H, Yakushev I, Siessmeier T, et al. Decreased dopamine D2/D3-receptor binding in temporal lobe epilepsy: an [18F]fallypride PET study. Epilepsia . 2006;47:1392–1396. doi: 10.1111/j.1528-1167.2006.00561.x. [DOI] [PubMed] [Google Scholar]
- 62.Hirose S. A new paradigm of channelopathy in epilepsy syndromes: Intracellular trafficking abnormality of channel molecules. Epilepsy Res. 2006;70S:S206–S217. doi: 10.1016/j.eplepsyres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 63.Graves TD. Ion channels and epilepsy. Q J Med . 2006;99:201–217. doi: 10.1093/qjmed/hcl021. [DOI] [PubMed] [Google Scholar]
- 64.Kass RS. The channelopathies: Novel insights into molecular and genetic mechanism of human disease. J Clin Invest . 2005;115:1986–1989. doi: 10.1172/JCI26011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirose S, Okada M, Kaneko S, Mitsudome A. Are some idiopathic epilepsies disorders of ion channels? A working hypothesis. Epilepsy Res . 2000;41:191–204. doi: 10.1016/s0920-1211(00)00141-8. [DOI] [PubMed] [Google Scholar]
- 66.Helbig I, Scheffer IE, Mulley JC, Berkovic SF. Navigating the channels and beyond: Unravelling the genetics of the epilepsies. Lancet Neurol . 2008;7:231–245. doi: 10.1016/S1474-4422(08)70039-5. [DOI] [PubMed] [Google Scholar]
- 67.Berkovic SF, Mulley JC, Scheffer IE, Petrou S. Human epilepsies: Interaction of genetic and acquired factors. Trends Neurosci . 2006;29:391–Trends Neurosci . doi: 10.1016/j.tins.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Scheffer IE, Berkovic SF. The genetics of human epilepsy. Trends Pharmacol Sci . 2003;24:428–433. doi: 10.1016/S0165-6147(03)00194-9. [DOI] [PubMed] [Google Scholar]
- 69.Richichi C, Brewster AL, Bender RA, Simeone TA, Zha Q, Yin HZ, et al. Mechanisms of seizure-induced ‘transcriptional channelopathy’ of hyperpolarization-activated cyclic nucleotide gated channels. Neurobiol Dis. 2008;29:297–305. doi: 10.1016/j.nbd.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuisle M, Wanaverbecq N, Brewster AL, Frere SGA, Pinault D, Baram TZ, et al. Functional stabilization of weakened thalamic pacemaker channel regulation in rat absence epilepsy. J Physiol . 2006;575:83–100. doi: 10.1113/jphysiol.2006.110486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poolos NP. The h-channel: A potential channelopathy in epilepsy? Epilepsy Behav. 2005;7:51–56. doi: 10.1016/j.yebeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 72.Bender RA, Baram TZ. Hyperpolarization activated cyclic-nucleotide gated (HCN) channels in developing neuronal networks. Prog Neurobiol . 2008;86:129–140. doi: 10.1016/j.pneurobio.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jung S, Jones TD, Lugo JN, Sheerin AH, Miller JW, D’Ambrosio R, et al. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol . 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- 75.Scharfmann HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: Functional implications of seizure-induced neurogenesis. J Neurosci . 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuruba R, Hattiangady B, Shetty AK. Hippocampal neurogenesis and neural stem cells in temporal lobe epilepsy. Epilepsy Behav . 2009;14:65–73. doi: 10.1016/j.yebeh.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cavazos JE, Golarai G, Sutula TP. Mossy fiber synaptic reorganization induced by kindling; Time course of development, progression and permanence. J Neurosci . 1991;11:2795–2803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sutula TP. Seizure-induced axonal sprouting: Assessing connections between injury, local circuits and epileptogenesis. Epilepsy Curr . 2002;2:86–91. doi: 10.1046/j.1535-7597.2002.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McNamara JO. Cellular and molecular basis of epilepsy. J Neurosci . 1994;14:3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: The “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus . 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- 81.Bekenstein JW, Lothman EW. Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science . 1993;259:97–100. doi: 10.1126/science.8093417. [DOI] [PubMed] [Google Scholar]
- 82.Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H. Regulation of brain-derived neurotropic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci . 1992;12:4793–4799. doi: 10.1523/JNEUROSCI.12-12-04793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suzuki F, Junier MP, Guilhem D, Sorensen JC, Onteniente B. Morphogenetic effect of kainate on adult hippocampal neurons associated with a prolonged expression of brain-derived neurotrophic factor. Neuroscience . 1995;64:665–674. doi: 10.1016/0306-4522(94)00463-f. [DOI] [PubMed] [Google Scholar]
- 84.Gomez-Pinilla F, van der Wal EA, Cotman CW. Possible coordinated geme expressions for FGF receptor, FGF-5 and FGF-2 following seizures. Exp Neurol . 1995;133:164–174. doi: 10.1006/exnr.1995.1019. [DOI] [PubMed] [Google Scholar]
- 85.Croll SD, Goodman JH, Scharfman HE. Vascular endothelial growth factor (VEGF) in seizures: a double-edged sword. Adv Exp Med Biol. 2004;548:57–68. doi: 10.1007/978-1-4757-6376-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Banerjee SB, Rajendran R, Dias BG, Ladiwala U, Tole S, Vaidya VA. Recruitment of the Sonic hedgehog signaling cascade in eletroconvulsive seizure-mediated regulation of adult rat hippocampal neurogenesis. Eur J Neurosci . 2005;22:1570–1580. doi: 10.1111/j.1460-9568.2005.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hopkins SJ, Rothwell NJ. Cytokines and the nervous system, I: Expression and recognition. Trends Neurosci . 1995;18:83–88. [PubMed] [Google Scholar]
- 88.Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Romanovitch AE, et al. Kindling modulates the IL-1β system, TNF-α, TGF-β1 and neuropeptide mRNAs in specific brain regions. Mol Brain Res. 2000;75:248–258. doi: 10.1016/s0169-328x(99)00306-x. [DOI] [PubMed] [Google Scholar]
- 89.Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Ravizza T, et al. Functional role of inflammatory cytokines and anti-inflammatory molecules in seizures and epileptogenesis. Epilepsia. 41:30–35. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- 90.Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis . 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 91.Vezzani A, Ravizza T, Balosso S, Aronica E. Glia as a source of cytokines: Implications for neuronal excitability and survival. Epilepsia . 2008;49:24–32. doi: 10.1111/j.1528-1167.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 92.Aronica E, Ravizza T, Zurolo E, Vezzani A. Astrocyte immune responses in epilepsy. Glia . 2012;60:1258–1268. doi: 10.1002/glia.22312. [DOI] [PubMed] [Google Scholar]
- 93.Nguyen MD, Julien JP, Rivest S. Innate immunity: The missing link in neuroprotection and neurodegeneration? . Nat Rev Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- 94.Vezzani A, Granata T. Brain inflammation in epilepsy: Experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 95.Vezzani A, Baram TZ. New roles for interleukin-1 beta in the mechanism of epilepsy. Epilepsy Curr . 2007;7:45–50. doi: 10.1111/j.1535-7511.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci . 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci . 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, et al. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, et al. Transcriptome profiling reveals TGF-β signaling involvement in epileptogenesis. J Neurosci . 2009;29:8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fabene PF, Mora GN, Martinello M, Rossi B, Merigo F, Ottoboni L, et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med . 2008;14:1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bursch W, Karwan A, Mayer M, Dornetshuber J, Frohwein U, Schulte-Hermann R, et al. Cell death and autophagy: Cytokines, drugs and nutritional factors. Toxicology . 2008;254:147–157. doi: 10.1016/j.tox.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 102.Liou AK, Clark RS, Henshall DC, Yin XM, Chen J. To die or not to die for neurons in ischemia, traumatic brain injury and epilepsy: A review on the stress-activated signaling pathways and apoptotic pathways. Prog Neurobiol . 2003;69:103–142. doi: 10.1016/s0301-0082(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 103.Narkilahti S, Pitkanen A. Caspase 6 expression in the rat hippocampus during epileptogenesis and epilepsy. Neuroscience . 2005;131:887–897. doi: 10.1016/j.neuroscience.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 104.Xu S, Pang Q, Liu Y, Shang W, Zhai G, Ge M. Neuronal apoptosis in the resected sclerotic hippocampus in patients with mesial temporal lobe epilepsy. J Clin Neurosci . 2007;14:835–840. doi: 10.1016/j.jocn.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 105.Moghadami M, Moghimi A, Jalal R, Behnam-Rosouli M, Mahdavi-Shahri S. Effects of infantile repeated hyperglycemia on neuronal density of hippocampus and pentylenetetrazol induced convulsions in male wistar rats. Iran J Basic Med Sci. 2012;15:951–957. [PMC free article] [PubMed] [Google Scholar]
- 106.Henshall DC, Simon RP. Epilepsy and apoptosis pathways. J Cereb Blood Flow Metab . 2005;25:1557–1572. doi: 10.1038/sj.jcbfm.9600149. [DOI] [PubMed] [Google Scholar]
- 107.Cory S, Adams JM. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 108.Albensi BC. Potential roles for tumor necrosis factor and nuclear factor-κB in seizure activity. J Neurosci Res . 2001;66:151–154. doi: 10.1002/jnr.1206. [DOI] [PubMed] [Google Scholar]
- 109.Narkilahti S, Pirttila TJ, Lukasiuk K, Tuunanen J, Pitkanen A. Expression and activation of caspase 3 following status epilepticus in the rat. Eur J Neurosci. 2003;18:1486–1496. doi: 10.1046/j.1460-9568.2003.02874.x. [DOI] [PubMed] [Google Scholar]
- 110.Weise J, Engelhorn T, Dorfler A, Aker S, Bahr M, Hufnagel A. Expression time course and spatial distribution of activated caspase-3 after experimental status epilepticus: Constribution of delayed neuronal cell death to seizure-induced neuronal injury. Neurobiol Dis. 2005;18:582–590. doi: 10.1016/j.nbd.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 111.Engel T, Henshall DC. Apoptosis, Bcl-2 family proteins and caspases: The ABCs of the seizure-damage and epileptogenesis? Int J Physiol Pathophysiol Pharmacol. 2009;1:97–115. [PMC free article] [PubMed] [Google Scholar]
- 112.Gass P, Herdegen T. Neuronal expression of AP-1 proteins in excitotoxic-neurodegerative disorders and following nerve fiber lesions. Progr Neurobiol. 1995;47:257–290. [PubMed] [Google Scholar]
- 113.Kovacs KJ. c-Fos as a transcription factor: A stressful (re)view from a functional map. Neurochem Int . 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 114.Beer J, Mielke K, Zipp M, Zimmermann M, Herdegen T. Expression of c-jun, junB, c-fos, fra-1 and fra-2 mRNA in the rat brain following seizure activity and axotomy. Brain Res . 1998;794:255–266. doi: 10.1016/s0006-8993(98)00233-9. [DOI] [PubMed] [Google Scholar]
- 115.Labiner DM, Butler LS, Cao Z, Hosford DA, Shin C, McNamara JO. Induction of c-fos mRNA by kindled seizures: Complex relationship with neuronal burst firing. J Neurosci . 1993;73:744–751. doi: 10.1523/JNEUROSCI.13-02-00744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gilby KL, Armstrong JN, Currie RW, Currie RW, Robertson HA. The effects of hypoxia-ischemia on expression of c-Fos, c-Jun and Hsp 70 in the young rat hippocampus. Brain Res Mol Brain Res . 1997;48:87–96. doi: 10.1016/s0169-328x(97)00085-5. [DOI] [PubMed] [Google Scholar]
- 117.Lee MC, Rho JL, Kim MK, Woo YJ, Kim JH, Nam SC, et al. c-JUN Expression and Apoptotic Cell Death in Kainate-Induced Temporal Lobe Epilepsy. J Korean Med Sci. 2001;16:649–656. doi: 10.3346/jkms.2001.16.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Herdegen T, Skene P, Bahr M. The c-Jun transcription factor – Biopotential mediator of neuronal death, survival and regeneration. Trends Neurosci . 1997;20:227–231. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]
- 119.Molina CA, Foulkes NS, Lalli E, Sassone-Corsi P. Inducible and negative autoregulation of CREM: an alternative promotor directs the expression of ICER, an early response repressor. Cell . 1993;75:875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- 120.Borlikova G, Endo S. Inducible cAMP early repressor (ICER) and brain functions. Mol Neurobiol . 2009;40:73–86. doi: 10.1007/s12035-009-8072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mioduszewska B, Jaworski J, Kaczmarek L. Inducible cAMP early repressor (ICER) in the nervous system: a transcriptional regulator of neuronal plasticity and programmed cell death. J Neurochem . 2003;87:1313–1320. doi: 10.1046/j.1471-4159.2003.02116.x. [DOI] [PubMed] [Google Scholar]
- 122.Jaworski J, Mioduszewska B, Sanchez-Capelo A, Figiel I, Habas A, Gozdz A, et al. Inducible cAMP early repressor, an endogenous antagonist of cAMP responsive element-binding protein, evokes neuronal apoptosis in vitro. J Neurosci . 2003;23:4519–4526. doi: 10.1523/JNEUROSCI.23-11-04519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Porter BE, Lund IV, Varodayan FP, Wallace RW, Blendy JA. The role of transcription factors cyclic-AMP responsive element modulator (CREM) and inducible cyclic-AMP early repressor (ICER) in epileptogenesis. Neuroscience . 2008;152:829–836. doi: 10.1016/j.neuroscience.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kojima N, Borlikova G, Sakamoto T, Yamada K, Ikeda T, Itohara S, et al. Inducible cAMP early repressor acts as a negative regulator for kindling epileptogenesis and long-term fear memory. J Neurosci . 2008;28:6459–6472. doi: 10.1523/JNEUROSCI.0412-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vezzani A, Schwarzer C, Lothman EW, Williamson J, Sperk G. Functional changes in somatostatin and neuropeptide Y containing neurons in the rat hippocampus in chronic models of limbic seizures. Epilepsy Res . 1996;26:267–279. doi: 10.1016/s0920-1211(96)00059-9. [DOI] [PubMed] [Google Scholar]
- 126.Schwarzer C, Williamson JM, Lothman EW, Vezzani A, Sperk G. Somatostatin, neuropeptide Y, neurokinin B and cholecystokinin immunoreactivity in two chronic models of temporal lobe epilepsy. Neuroscience . 1995;69:831–845. doi: 10.1016/0306-4522(95)00268-n. [DOI] [PubMed] [Google Scholar]
- 127.Wilson DN, Chung H, Elliott RC, Bremer E, George D, Koh S. Microarray analysis of postictal transcriptional regulation of neuropeptides. J Mol Neurosci . 2005;25:285–297. doi: 10.1385/JMN:25:3:285. [DOI] [PubMed] [Google Scholar]
- 128.Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci . 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- 129.Vezzani A, Sperk G. Overexpression of NPY and Y2 receptors in epileptic brain tissue: An endogenous neuroprotective mechanism in temporal lobe epilepsy? . Neuropeptides. 2004;38:245–252. doi: 10.1016/j.npep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 130.Lurton D, Coussemacq M, Barrow P, Sundstrom LE, Rougier A. Widespread ectopic neuropeptide-Y immunoreactivity mossy fibers after a unilateral intrahippocampal kainic in the rat in contralateral acid injection. Neurosci Lett . 1996;213:181–184. doi: 10.1016/0304-3940(96)12854-8. [DOI] [PubMed] [Google Scholar]
- 131.Chafetz RS, Nahm WK, Noebels JL. Aberrant expression of neuropeptide Y in hippocampal mossy fibers in the absence of local cell injury following the onset of spike-wave synchronization. Mol Brain Res . 1995;31:111–121. doi: 10.1016/0169-328x(95)00041-p. [DOI] [PubMed] [Google Scholar]
- 132.Noe F, Frasca A, Balducci C, Carli M, Sperk G, Ferraguti F, et al. Neuropeptide Y overexpression using recombinant adenoassociated viral vectors. Neurotherapeutics . 2009;6:300–306. doi: 10.1016/j.nurt.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Noe F, Pool AH, Nissinen J, Gobbi M, Bland R, Rizzi M, et al. Neuropeptide Y gene therapy decreases chronic spontaneous seizures in a rat model of temporal lobe epilepsy. Brain . 2008;131:1506–1515. doi: 10.1093/brain/awn079. [DOI] [PubMed] [Google Scholar]
- 134.Mazarati AM, Liu H, Soomets U, Sankar R, Shin D, Katsumori H, et al. Galanin modulation of seizures and seizure modulation of hippocampal galanin in animal models of status epilepticus. J Neurosci . 1998;18:10070–10077. doi: 10.1523/JNEUROSCI.18-23-10070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mazarati AM. Galanin and galanin receptors in epilepsy. Neuropeptides . 2004;38:331–343. doi: 10.1016/j.npep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 136.Mazarati A, Lu X. Regulation of limbic status epilepticus by hippocampal galanin type 1 and type 2 receptors. Neuropeptides . 2005;39:277–280. doi: 10.1016/j.npep.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 137.Mazarati A, Lundstrom L, Sollenberg U, Shin D, Langel U, Sankar R. Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: the effects of subtype-selective agonists and the role of G-protein-mediated signaling. J Pharmacol Exp Ther . 2006;318:700–708. doi: 10.1124/jpet.106.104703. [DOI] [PubMed] [Google Scholar]
- 138.Kanter-Schlifke I, Toft Sorensen A, Ledri M, Kuteeva E, Hokfelt T, Kokaia M. Galanin gene transfer curtails generalized seizures in kindled rats without altering hippocampal synaptic plasticity. Neuroscience . 2007;150:984–992. doi: 10.1016/j.neuroscience.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 139.Stables JP, Bertram E, Dudek FE, Holmes G, Mathern G, Pitkanen A, et al. Therapy discovery for pharmacoresistant epilepsy and for disease modifying therapeutics: Summary of the NIH/NINDS/AES Models II Workshop. Epilepsia . 2003;44:1472–1478. doi: 10.1111/j.0013-9580.2003.32803.x. [DOI] [PubMed] [Google Scholar]
- 140.Pitkanen A, Kharatishvili I, Karhunen H, Lukasiuk K, Immonen R, Nairismagi J, et al. Epileptogenesis in experimental models. Epilepsia . 2007;48:13–20. doi: 10.1111/j.1528-1167.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 141.Stables JP, Bertnam EH, White HS, Coulter DA, Dichter MA, Jacobs MP, et al. Models of epilepsy and epileptogenesis: Report from NIH workshop, Bethesda, Maryland. Epilepsia. 2002;43:1410–1420. doi: 10.1046/j.1528-1157.2002.06702.x. [DOI] [PubMed] [Google Scholar]
- 142.McNamara JO, Bonhaus DW, Shin C. New York: Cambridge University Press; 1993. The kindling model of epilepsy; pp. 27–47. In: Schwartzkroin PA, editor. Epilepsy: Models, mechanism and concepts. [Google Scholar]
- 143.McIntyre DC, Poulter MO, Gilby K. Kindling: Some old and some new. Epilepsy Res . 2002;50:79–92. doi: 10.1016/s0920-1211(02)00071-2. [DOI] [PubMed] [Google Scholar]
- 144.Gupta YK, Veerendra Kumar MH, Srivastava AK. Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacol Biochem Behav. 2003;74:579–585. doi: 10.1016/s0091-3057(02)01044-4. [DOI] [PubMed] [Google Scholar]
- 145.Silva LF, Pereira P, Elisabetsky E. A neuropharmacological analysis of PTZ-induced kindling in mice. Gen Pharm . 1998;31:47–50. doi: 10.1016/s0306-3623(97)00423-0. [DOI] [PubMed] [Google Scholar]
- 146.Nissinen J, Halonen T, Koivisto E, Pitkanen A. A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rat. Epilepsy Res . 2000;38:177–205. doi: 10.1016/s0920-1211(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 147.Covolan L, Mello LE. Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res . 2000;39:133–152. doi: 10.1016/s0920-1211(99)00119-9. [DOI] [PubMed] [Google Scholar]
- 148.Leite JP, Garcia-Cairasco N, Cavalheiro EA. New sights from the use of pilocarpine and kainate models. Epilepsy Res . 2002;50 doi: 10.1016/s0920-1211(02)00072-4. [DOI] [PubMed] [Google Scholar]
- 149.Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Reccurent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: Assessment of a rat model of temporal lobe epilepsy. Epilepsy Res . 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- 150.Sloviter R. Hippocampal epileptogenesis in animal models of mesial temporal lobe epilepsy with hippocampal sclerosis: The importance of the ‘latent period’ and other concepts. Epilepsia . 2008;49:85–92. doi: 10.1111/j.1528-1167.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 151.Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW. Mesial temporal lobe epilepsy: Pathogenesis, induced rodent models and lesions. Toxicol Pathol . 2007;35:984–998. doi: 10.1080/01926230701748305. [DOI] [PubMed] [Google Scholar]
- 152.Fritschy M. A new animal model of temporal lobe epilepsy. Epileptologie . 2004;21:21–28. [Google Scholar]
- 153.Marcangelo MJ, Ovsiew F. Psychiatric aspects of epilepsy. Psychiatr Clin North Am . 2007;30:781–802. doi: 10.1016/j.psc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 154.Grotickle I, Hoffmann K, Loscher W. Behavioral alterations in the pilocarpine model of temporal lobe epilepsy in mice. Exp Neurol . 2007;207:329–349. doi: 10.1016/j.expneurol.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 155.McCord MC, Lorenzana A, Bloom CS, Chancer ZO, Schauwecker PE. Effect of age on kainate-induced seizure severity and cell death. Neuroscience . 2008;154:1143–1153. doi: 10.1016/j.neuroscience.2008.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brandt C, Glien M, Potschka H, Volk H, Loscher W. Epileptogenesis and neuropathology after different types of status epilepticus induced by prolonged electrical stimulation of the basolateral amygdala in rats. Epilepsy Res . 2003;55:83–103. doi: 10.1016/s0920-1211(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 157.Haas KZ, Sperber EF, Opanashuk LA, Stanton PK, Moshe SL. Resistance of immature hippocampus to morphologic and physiologic alteration following status epilepticus or kindling. Hippocampus . 2001;11:615–625. doi: 10.1002/hipo.1076. [DOI] [PubMed] [Google Scholar]
- 158.Schauwecker PE. The relevance of individual genetic background and its role in animal models of epilepsy. Epilepsy Res . 2011;97:1–11. doi: 10.1016/j.eplepsyres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cole AJ, Koh S, Zheng Y. Are seizures harmful: What can we learn from animal models? . Prog Brain Res. 2002;135:13–23. doi: 10.1016/S0079-6123(02)35004-0. [DOI] [PubMed] [Google Scholar]