Abstract
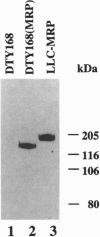
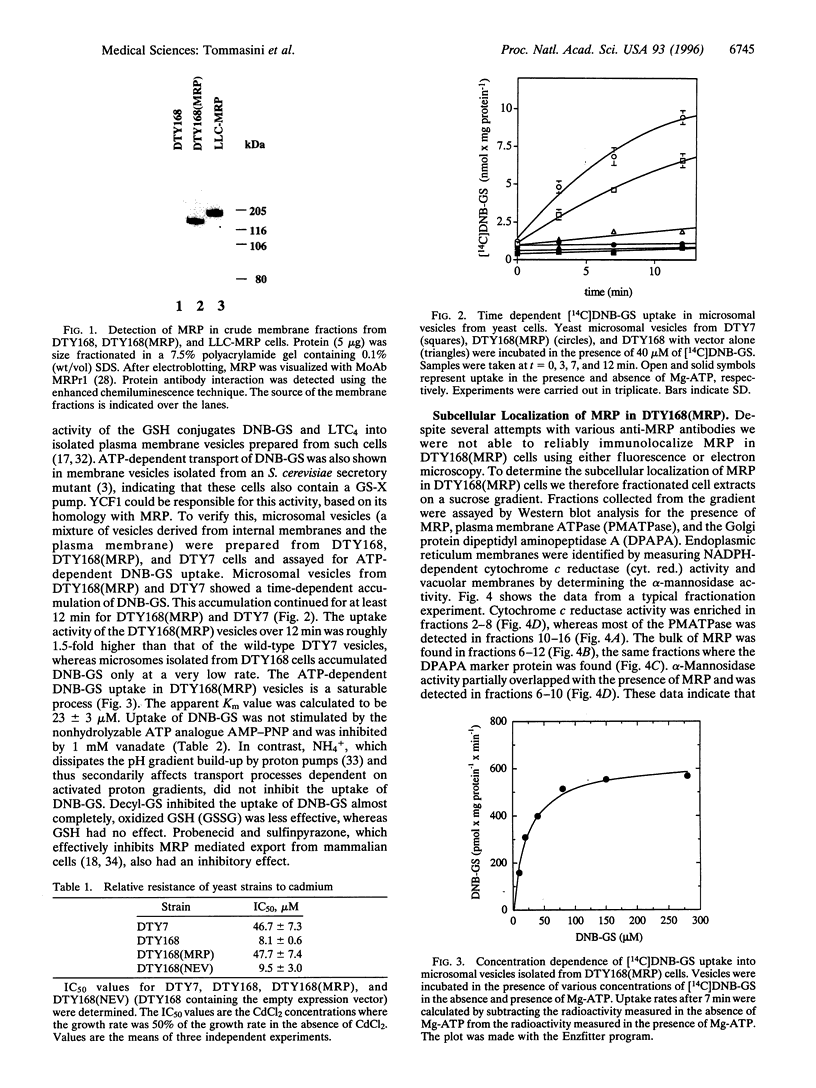
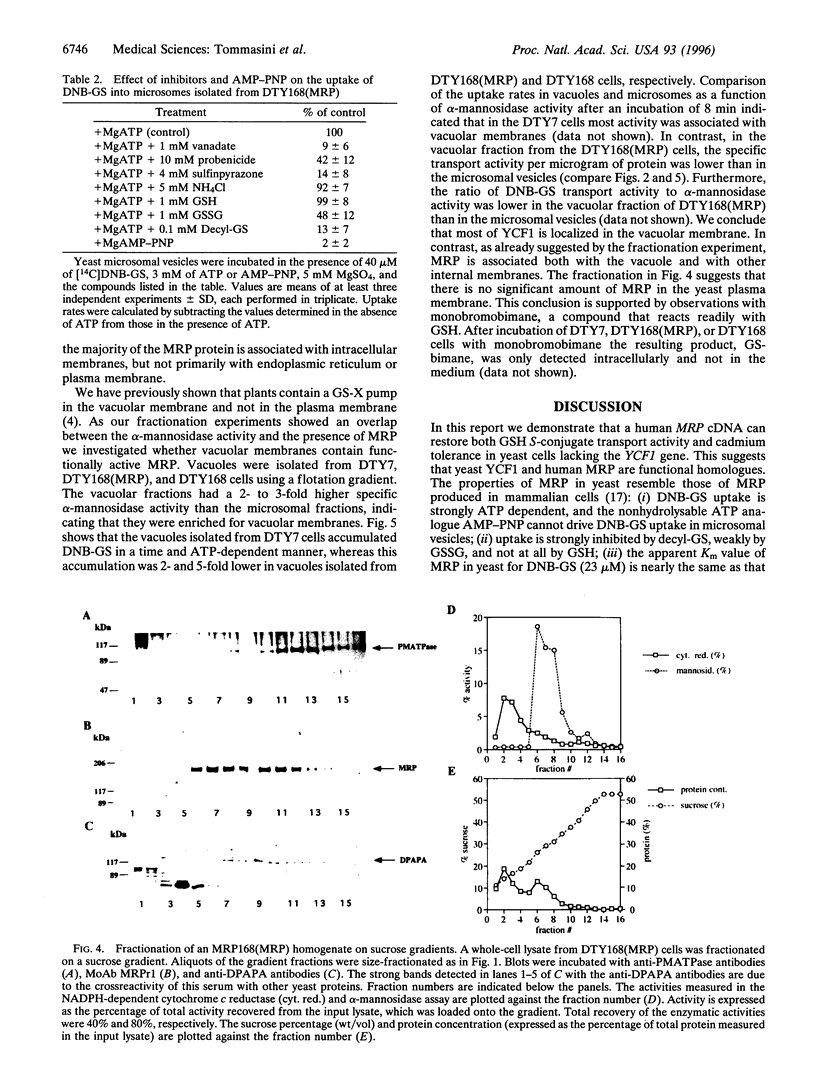
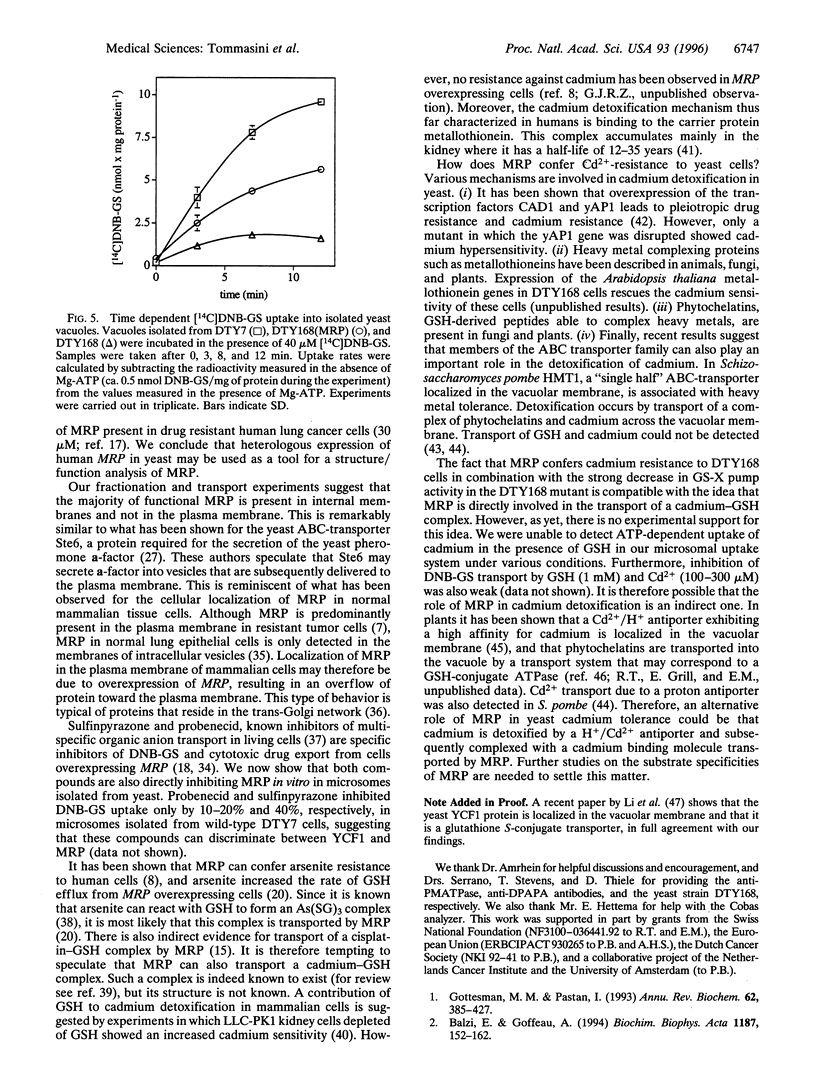
A Saccharomyces cerevisiae strain with a disrupted yeast cadmium resistance factor (YCF1) gene (DTY168) is hypersensitive to cadmium. YCF1 resembles the human multidrug resistance-associated protein MRP (63% amino acid similarity), which confers resistance to various cytotoxic drugs by lowering the intracellular drug concentration. Whereas the mechanism of action of YCF1 is not known, MRP was recently found to transport glutathione S-conjugates across membranes. Here we show that expression of the human MRP cDNA in yeast mutant DTY168 cells restores cadmium resistance to the wild-type level. Transport of S-(2,4-dinitrobenzene)-glutathione into isolated yeast microsomal vesicles is strongly reduced in the DTY168 mutant and this transport is restored to wild-type level in mutant cells expressing MRP cDNA. We find in cell fractionation experiments that YCF1 is mainly localized in the vacuolar membrane in yeast, whereas MRP is associated both with the vacuolar membrane and with other internal membranes in the transformed yeast cells. Our results indicate that yeast YCF1 is a glutathione S-conjugate pump, like MRP, and they raise the possibility that the cadmium resistance in yeast involves cotransport of cadmium with glutathione derivatives.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balzi E., Goffeau A. Genetics and biochemistry of yeast multidrug resistance. Biochim Biophys Acta. 1994 Aug 30;1187(2):152–162. doi: 10.1016/0005-2728(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Sparks K. E., Fraser K., Loe D. W., Grant C. E., Wilson G. M., Deeley R. G. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994 Nov 15;54(22):5902–5910. [PubMed] [Google Scholar]
- Delnomdedieu M., Basti M. M., Otvos J. D., Thomas D. J. Reduction and binding of arsenate and dimethylarsinate by glutathione: a magnetic resonance study. Chem Biol Interact. 1994 Feb;90(2):139–155. doi: 10.1016/0009-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Fasolato C., Steinberg T. H. Inhibitors of membrane transport system for organic anions block fura-2 excretion from PC12 and N2A cells. Biochem J. 1988 Dec 15;256(3):959–963. doi: 10.1042/bj2560959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers R., Zaman G. J., van Deemter L., Jansen H., Calafat J., Oomen L. C., Oude Elferink R. P., Borst P., Schinkel A. H. Basolateral localization and export activity of the human multidrug resistance-associated protein in polarized pig kidney cells. J Clin Invest. 1996 Mar 1;97(5):1211–1218. doi: 10.1172/JCI118535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller N., Broxterman H. J., Währer D. C., Pinedo H. M. ATP-dependent efflux of calcein by the multidrug resistance protein (MRP): no inhibition by intracellular glutathione depletion. FEBS Lett. 1995 Jul 17;368(2):385–388. doi: 10.1016/0014-5793(95)00677-2. [DOI] [PubMed] [Google Scholar]
- Flens M. J., Izquierdo M. A., Scheffer G. L., Fritz J. M., Meijer C. J., Scheper R. J., Zaman G. J. Immunochemical detection of the multidrug resistance-associated protein MRP in human multidrug-resistant tumor cells by monoclonal antibodies. Cancer Res. 1994 Sep 1;54(17):4557–4563. [PubMed] [Google Scholar]
- Flens M. J., Zaman G. J., van der Valk P., Izquierdo M. A., Schroeijers A. B., Scheffer G. L., van der Groep P., de Haas M., Meijer C. J., Scheper R. J. Tissue distribution of the multidrug resistance protein. Am J Pathol. 1996 Apr;148(4):1237–1247. [PMC free article] [PubMed] [Google Scholar]
- Fujii R., Mutoh M., Sumizawa T., Chen Z. S., Yoshimura A., Akiyama S. Adenosine triphosphate-dependent transport of leukotriene C4 by membrane vesicles prepared from cisplatin-resistant human epidermoid carcinoma tumor cells. J Natl Cancer Inst. 1994 Dec 7;86(23):1781–1784. doi: 10.1093/jnci/86.23.1781. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Hörtensteiner S., Vogt E., Hagenbuch B., Meier P. J., Amrhein N., Martinoia E. Direct energization of bile acid transport into plant vacuoles. J Biol Chem. 1993 Sep 5;268(25):18446–18449. [PubMed] [Google Scholar]
- Ishikawa T. ATP/Mg2+-dependent cardiac transport system for glutathione S-conjugates. A study using rat heart sarcolemma vesicles. J Biol Chem. 1989 Oct 15;264(29):17343–17348. [PubMed] [Google Scholar]
- Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci. 1992 Nov;17(11):463–468. doi: 10.1016/0968-0004(92)90489-v. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Wright C. D., Ishizuka H. GS-X pump is functionally overexpressed in cis-diamminedichloroplatinum (II)-resistant human leukemia HL-60 cells and down-regulated by cell differentiation. J Biol Chem. 1994 Nov 18;269(46):29085–29093. [PubMed] [Google Scholar]
- Jedlitschky G., Leier I., Buchholz U., Center M., Keppler D. ATP-dependent transport of glutathione S-conjugates by the multidrug resistance-associated protein. Cancer Res. 1994 Sep 15;54(18):4833–4836. [PubMed] [Google Scholar]
- Keppler D. Leukotrienes: biosynthesis, transport, inactivation, and analysis. Rev Physiol Biochem Pharmacol. 1992;121:1–30. doi: 10.1007/BFb0033192. [DOI] [PubMed] [Google Scholar]
- Kim E. J., Zhen R. G., Rea P. A. Heterologous expression of plant vacuolar pyrophosphatase in yeast demonstrates sufficiency of the substrate-binding subunit for proton transport. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6128–6132. doi: 10.1073/pnas.91.13.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölling R., Hollenberg C. P. The ABC-transporter Ste6 accumulates in the plasma membrane in a ubiquitinated form in endocytosis mutants. EMBO J. 1994 Jul 15;13(14):3261–3271. doi: 10.1002/j.1460-2075.1994.tb06627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leier I., Jedlitschky G., Buchholz U., Cole S. P., Deeley R. G., Keppler D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem. 1994 Nov 11;269(45):27807–27810. [PubMed] [Google Scholar]
- Li Z. S., Szczypka M., Lu Y. P., Thiele D. J., Rea P. A. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J Biol Chem. 1996 Mar 15;271(11):6509–6517. doi: 10.1074/jbc.271.11.6509. [DOI] [PubMed] [Google Scholar]
- Li Z. S., Zhao Y., Rea P. A. Magnesium Adenosine 5[prime]-Triphosphate-Energized Transport of Glutathione-S-Conjugates by Plant Vacuolar Membrane Vesicles. Plant Physiol. 1995 Apr;107(4):1257–1268. doi: 10.1104/pp.107.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Meijer C., Zaman G. J., Borst P., Scheper R. J., Mulder N. H., de Vries E. G., Jansen P. L. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):13033–13037. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto R. K., Rao R., Slayman C. W. Expression of the yeast plasma membrane [H+]ATPase in secretory vesicles. A new strategy for directed mutagenesis. J Biol Chem. 1991 Apr 25;266(12):7940–7949. [PubMed] [Google Scholar]
- Nilsson T., Warren G. Retention and retrieval in the endoplasmic reticulum and the Golgi apparatus. Curr Opin Cell Biol. 1994 Aug;6(4):517–521. doi: 10.1016/0955-0674(94)90070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1981 Mar 10;256(5):2079–2082. [PubMed] [Google Scholar]
- Ortiz D. F., Kreppel L., Speiser D. M., Scheel G., McDonald G., Ow D. W. Heavy metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO J. 1992 Oct;11(10):3491–3499. doi: 10.1002/j.1460-2075.1992.tb05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D. F., Ruscitti T., McCue K. F., Ow D. W. Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J Biol Chem. 1995 Mar 3;270(9):4721–4728. doi: 10.1074/jbc.270.9.4721. [DOI] [PubMed] [Google Scholar]
- Prozialeck W. C., Lamar P. C. Effects of glutathione depletion on the cytotoxic actions of cadmium in LLC-PK1 cells. Toxicol Appl Pharmacol. 1995 Oct;134(2):285–295. doi: 10.1006/taap.1995.1194. [DOI] [PubMed] [Google Scholar]
- Ruetz S., Gros P. Functional expression of P-glycoproteins in secretory vesicles. J Biol Chem. 1994 Apr 22;269(16):12277–12284. [PubMed] [Google Scholar]
- Salt D. E., Rauser W. E. MgATP-Dependent Transport of Phytochelatins Across the Tonoplast of Oat Roots. Plant Physiol. 1995 Apr;107(4):1293–1301. doi: 10.1104/pp.107.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt D. E., Wagner G. J. Cadmium transport across tonoplast of vesicles from oat roots. Evidence for a Cd2+/H+ antiport activity. J Biol Chem. 1993 Jun 15;268(17):12297–12302. [PubMed] [Google Scholar]
- Sauer N., Stolz J. SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker's yeast and identification of the histidine-tagged protein. Plant J. 1994 Jul;6(1):67–77. doi: 10.1046/j.1365-313x.1994.6010067.x. [DOI] [PubMed] [Google Scholar]
- St-Pierre M. V., Ruetz S., Epstein L. F., Gros P., Arias I. M. ATP-dependent transport of organic anions in secretory vesicles of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9476–9479. doi: 10.1073/pnas.91.20.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R., Wolf K., Hilgarth C., Tanner W., Sauer N. Subcellular localization of the inducible Chlorella HUP1 monosaccharide-H+ symporter and cloning of a Co-induced galactose-H+ symporter. Plant Physiol. 1995 Jan;107(1):33–41. doi: 10.1104/pp.107.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczypka M. S., Wemmie J. A., Moye-Rowley W. S., Thiele D. J. A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein. J Biol Chem. 1994 Sep 9;269(36):22853–22857. [PubMed] [Google Scholar]
- Versantvoort C. H., Broxterman H. J., Bagrij T., Scheper R. J., Twentyman P. R. Regulation by glutathione of drug transport in multidrug-resistant human lung tumour cell lines overexpressing multidrug resistance-associated protein. Br J Cancer. 1995 Jul;72(1):82–89. doi: 10.1038/bjc.1995.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Wemmie J. A., Edgington N. P., Goebl M., Guevara J. L., Moye-Rowley W. S. Yeast bZip proteins mediate pleiotropic drug and metal resistance. J Biol Chem. 1993 Sep 5;268(25):18850–18858. [PubMed] [Google Scholar]
- Zaman G. J., Flens M. J., van Leusden M. R., de Haas M., Mülder H. S., Lankelma J., Pinedo H. M., Scheper R. J., Baas F., Broxterman H. J. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8822–8826. doi: 10.1073/pnas.91.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman G. J., Lankelma J., van Tellingen O., Beijnen J., Dekker H., Paulusma C., Oude Elferink R. P., Baas F., Borst P. Role of glutathione in the export of compounds from cells by the multidrug-resistance-associated protein. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7690–7694. doi: 10.1073/pnas.92.17.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]