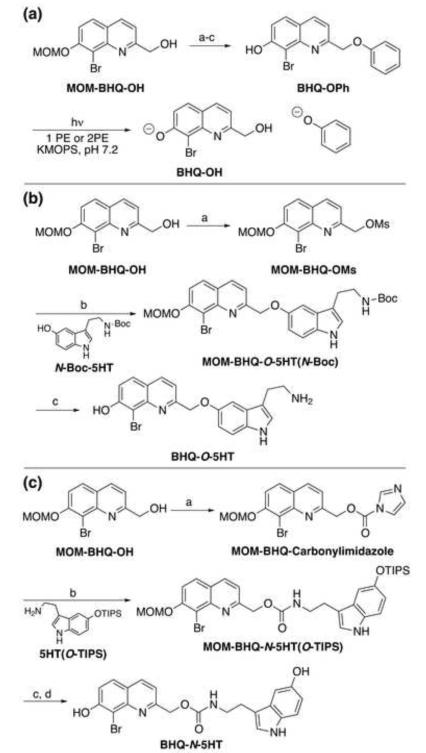
Figure 2.
(a) Preparation and photolysis of BHQ-OPh. (a) MsCl, DIEA, THF, rt, 2 h, 68%; (b) phenol, 1 M KOH (aq.), THF, 72%; (c) TFA, MeOH. Time courses for the photolysis of BHQ-OPh are shown in Figure S1. (b) Preparation of BHQ-O-5HT. (a) MsCl, DIEA, THF, rt, 2 h, 68%; (b) K2CO3, CH3CN, reflux, 48 h, 78%; (c) TFA, CH2Cl2, rt, 1 h, 57%. (c) Preparation of BHQ-N-5HT. (a) carbonyldiimidazole, THF, rt, 2 h, 63%; (b) DMF, 60 ○C, 12 h, 65%; (c) TBAF, THF, rt 15 min, 85%; (d) conc. HCl (tr), MeOH, rt, 12 h, 55%.