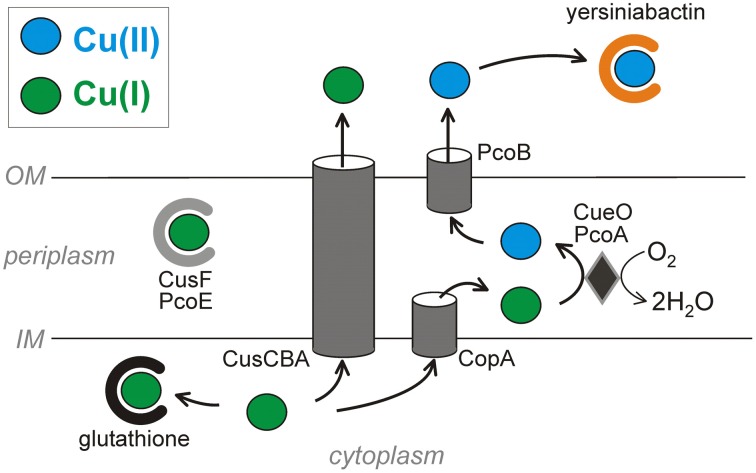
Figure 1.
Copper resistance strategies across pathogenic E. coli membranes. The virulence-associated siderophore yersiniabactin sequesters Cu2+ outside the cell and prevents its reduction to the more toxic Cu+. Copper ions that reach the cytosol are subject to chelation by glutathione and export by two ATPases. The CusCBA ATPase complex exports Cu+ from both the cytoplasm and the periplasm (via CusF) to the extracellular space. Alternatively, the CopA ATPase exports cytoplasmic copper across the inner membrane. Periplasmic Cu+ can bind the proteins CusF and PcoE or be oxidized by the mixed copper oxidases CueO or PcoA to less toxic Cu2+. PcoB has a putative function of exporting Cu2+ across the outer membrane. The systems are oriented to minimize free cytosolic copper ions by directing these to the periplasmic or extracellular spaces.